Researchers uncover role of A-site cation ordering in perovskite anodes for high-temperature oxygen evolution

Lisa Lock
scientific editor

Robert Egan
associate editor

Solid oxide electrolysis cells (SOECs) are a leading technology for carbon dioxide reduction and energy conversion, offering high current densities, excellent Faradaic efficiency, and low overpotentials. Perovskite oxides are commonly used as SOEC anodes, yet the impact of A-site cation ordering on their oxygen evolution reaction (OER) kinetics remains unexplored.
In a study in the Journal of the American Chemical Society, Assoc. Prof. Song Yuefeng and colleagues from the Dalian Institute of Chemical Â鶹ÒùÔºics (DICP) of the Chinese Academy of Sciences, collaborating with Prof. Wang Guoxiong from Fudan University and Prof. Liu Meilin from Georgia Institute of Technology, uncovered the mechanisms underlying the anodic high-temperature OER in SOECs.
Researchers focused on how A-site cation ordering affects the electrocatalytic performance of perovskite anodes, and particularly examined the order–disorder transition in PrxBa2-xCo2O5+δ.
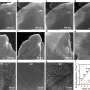
Researchers synthesized two perovskite anodes with different Pr contents, PrBaCo2O5+δ (PBCO-1.0) and Pr1.5Ba0.5Co2O5+δ (PBCO-1.5), and systematically investigated the effect of A-site cation ordering on the electronic structure and high-temperature OER kinetics.
They found that as the Pr content increased from 1.0 to 1.5, the crystal structure transitioned from an ordered tetragonal phase (P4/mmm) to a disordered orthorhombic phase (Pnma). This structural transformation disrupted the local symmetry of the Co–O coordination. It enhanced the orbital hybridization between Co 3d and O 2p states, and improved oxygen ion mobility, ultimately accelerating surface oxygen exchange.
At 800°C and 1.6 V, the PBCO-1.5 anode delivered a high current density of 2.29 A cm-2, demonstrating good high-temperature OER activity and stability.
"Our study combines experimental data with theoretical insights to show how A-site cation ordering in perovskite oxides governs the reaction pathway and kinetics of high-temperature OER. The findings provide valuable guidance for the rational design of high-performance SOEC anodes," said Assoc. Prof. Song.
More information: Lina Yu et al, Breaking the Ion Ordering in the Perovskite Anode for Enhanced High-Temperature Oxygen Evolution Reaction Activity, Journal of the American Chemical Society (2025).
Journal information: Journal of the American Chemical Society
Provided by Chinese Academy of Sciences