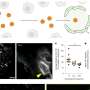
The proposed theory in this study explains the complex size- and time-dependent growth of nanoparticles, representing a fundamental shift in nanoparticle research. Credit: Ph.D. student Jingyu Kang, Dr. Ji-Hyun Kim, and Professor Jaeyoung Sung / Chung-Ang University
Nanoparticles have diverse applications in modern science and industry, powering technologies like quantum-dot displays, nanocatalysts and drug delivery. Their unique physicochemical properties, which can be tuned by changing their size and shape, make them highly attractive.
However, despite extensive research, the exact mechanisms and dynamics of monodisperse, or uniformly sized, nanoparticle formation and growth remain poorly understood.
The classical nucleation theory (CNT), based on the Gibbs-Thomson equation, has been the primary framework for understanding nanoparticle growth for over a century. However, this theory cannot explain why nanoparticle systems settle into uniform size ranges.
Recent studies have employed liquid-phase transmission electron microscopy (TEM) of individual nanoparticles to reveal the complex nature of nanoparticle growth dynamics. Yet, even before the advent of such cutting-edge techniques, a quantitative understanding of earlier experimental observations on nanoparticle growth dynamics had remained elusive until now.
In a breakthrough study, a theory team led by Professor Jaeyoung Sung from the Department of Chemistry and Global Science Research Center for Systems Chemistry at Chung-Ang University in South Korea have developed a new model and theory to explain the multiphasic growth dynamics of nanoparticle ensembles.
"Real-time, in-situ growth trajectories of nanoparticle ensembles, obtained by our liquid TEM experiment, motivated Professor Sung to develop a new theory of growing nanoparticle systems," explains Professor Jungwon Park from Seoul National University.
"This theory marks a fundamental shift in our understanding of nanoparticle formation and time evolution," said Distinguished Professor Taeghwan Hyeon, the Director of IBS Center for Nanoparticle Research, South Korea.
Professor Jungwon Park and Professor Taeghwan Hyeon led the experimental research in this collaboration. They are leading experts in liquid-phase TEM and nanoparticle synthesis, respectively. This multidisciplinary study was published in .
Using the liquid-phase TEM, the researchers directly observed the growth trajectories of hundreds of colloidal nanoparticles, a few nanometers in size, in real time. The results revealed that nanoparticles exhibited complex size-dependent growth dynamics with multiple kinetic phases.
In each of these kinetic phases, the statistics of nanoparticle size and their size-dependent growth showed distinct variations. They also found that nanoparticles undergo coalescence only in a small localized time window. These observations are unexplainable by previously reported theories.
Based on these findings, the team developed a new model and theory for nanoparticle growth. This model accounts for six essential characteristics of nanoparticle growth, including the nanoparticles' energy, shape, configurational degeneracy, monomer's diffusion coefficient, and the monomer association rate on the nanoparticle surface.
The new theory also accounts for translation, rotation and vibration of a nanoparticle, as well as its interaction with surrounding molecules, factors that were overlooked in the CNT.
As a result, this new theory provides fresh physical insights into the role of nanoparticle motion and configurational degeneracy in their nucleation and growth, along with an unprecedented quantitative explanation of experimental data for nanoparticle growth dynamics.
It also has broad applicability, validated across diverse nanoparticles, including platinum nanoparticles synthesized using different precursors, as well as metal oxide and semiconductor nanoparticles, under varying experimental conditions.
Interestingly, this theory predicts that smaller nanoparticles can grow while larger ones dissolve, which is in direct contradiction with the classical Ostwald ripening picture, a remarkable new insight that explains why nanoparticle systems exhibit uniform size distributions and size-focusing dynamics.
"This work makes it possible to understand time-dependent size distributions of nanoparticles and their size-dependent growth dynamics in terms of fundamental principles in physics and chemistry," said Professor Sung.
"This general theory can also be used to understand biological condensate formation and aggregation, which occur in many neurodegenerative diseases, including Alzheimer's disease."
"However, understanding is one thing, and prediction is another. Together with advances in artificial intelligence and computational chemistry, our theory offers a new framework for predictable nanoparticle synthesis, representing an exciting new direction for nanoparticle research. This knowledge will prove useful for developing tailored nanoparticles for industrial applications like catalyst design, semiconductor manufacturing, and drug delivery," concludes Professor Sung.
More information: Ji-Hyun Kim et al, Multiphasic size-dependent growth dynamics of nanoparticle ensembles, Proceedings of the National Academy of Sciences (2025).
Journal information: Proceedings of the National Academy of Sciences
Provided by Chung Ang University