Molecular hybridization achieved through quantum vacuum manipulation

Gaby Clark
scientific editor

Robert Egan
associate editor

Interactions between atoms and molecules are facilitated by electromagnetic fields. The bigger the distance between the partners involved, the weaker these mutual interactions are. In order for the particles to be able to form natural chemical bonds, the distance between them must usually be approximately equal to their diameter.
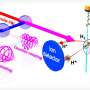
Using an optical resonator which strongly alters the quantum vacuum, scientists at the Max Planck Institute for the Science of Light (MPL) have succeeded for the first time in optically "bonding" several molecules at greater distances. The physicists are thus experimentally creating synthetic states of coupled molecules, thereby establishing the foundation for the development of new hybrid light-matter states. The study is in the journal Proceedings of the National Academy of Sciences.
Atoms and molecules have clearly defined, discrete energy levels. When they are combined to form a new molecule, the energy states change. This process is referred to as molecular hybridization and is characterized by the overlap of electron orbitals, i.e., the areas where electrons typically reside. However, at a scale of a few nanometers, the interaction becomes so weak that molecules are no longer able to communicate with each other.
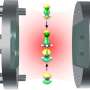
A team led by Professor Vahid Sandoghdar, director at MPL and head of the "Nano-Optics" Division, has succeeded for the first time in coupling spatially separated molecules via a modified vacuum field in an optical microresonator.
In the interior of a high-quality plano-concave microresonator, that is, between two mirrors of exceptional quality, light can be stored for an extended period of time. The scientists inserted an anthracene microcrystal doped with specific dye molecules into the resonator, which is only a few micrometers across. Utilizing high-resolution laser spectroscopy, the team then investigated the interaction of the molecules and their hybridization with the resonator mode.
The emergence of new features in the resulting spectrum indicates alterations to the molecular energy states, such as so-called subradiant and superradiant modes. Subradiant states emit less strongly than before, while superradiant states interact more strongly with light.
A notable consequence of the hybridization of two molecules is that they can then be elevated to the excited state concurrently. This means that they are no longer completely independent of each other. To achieve this, two photons are absorbed from the resonator. In this work there is for the first time a two-photon excitation of two molecules that are far apart. While alone, each photon shows no effect—but together they activate both molecules simultaneously. Neither the molecules nor the photons can act alone—but in harmony, they succeed.
Sandoghdar says, "Quantum states are usually very fragile, so it's a challenge to couple multiple molecules together. Our work establishes the foundation for the development of novel states in which material particles, such as molecules, are 'glued' together with light. The investigation of a precisely defined number of interacting emitters is also an important building block for the processing of quantum information and therefore of great interest in quantum technology."
More information: Jahangir Nobakht et al, Hybridization of molecules via a common photonic mode, Proceedings of the National Academy of Sciences (2025).
Journal information: Proceedings of the National Academy of Sciences
Provided by Max Planck Institute for the Science of Light