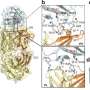
(A) Structural propagation pathways originating from galactose residues within the Fc-linked glycan. (B) Galactose residues (indicated by yellow circles) suppress glycan mobility by acting as molecular anchors, and constrain Fc domain dynamics by serving as molecular wedges—together enhancing the stability of the functional site and promoting effector molecule binding. Credit: Saeko Yanaka, Koichi Kato
A collaborative team from the Exploratory Research Center on Life and Living Systems (ExCELLS), National Institutes of Natural Sciences, and affiliated institutions has elucidated how glycosylation dynamically modulates the architecture of human IgG antibodies.
The study, in the Proceedings of the National Academy of Sciences , focuses on the Fc region of IgG1 and its glycoform-dependent behavior.
Using stable-isotope-assisted NMR spectroscopy and molecular dynamics simulations, the researchers analyzed four distinct glycoforms of IgG1-Fc, varying in galactose and fucose content. They discovered that galactose residues act as molecular "anchors" and "wedges," stabilizing the Fc domain and enhancing its interactions with immune effector molecules such as Fcγ receptors and complement C1q.
In contrast, the absence of core fucose reshaped the dynamics of key amino acid residues involved in antibody-dependent cellular cytotoxicity (ADCC).
The concept of "molecular meridians" emerged from the observation that glycan-induced structural changes propagate through the Fc region, connecting distant domains and influencing overall antibody behavior. Much like acupuncture meridians in traditional medicine, these molecular pathways transmit structural signals across the antibody, fine-tuning its immune functions.
This study highlights the power of integrating experimental and computational approaches to decode the dynamic programming of glycoproteins—revealing how structural signals from glycans shape antibody behavior.
These atomic-level insights into glycosylation open new avenues for glycoengineering, enabling the design of antibodies with optimized clinical performance for cancer, autoimmune diseases, and infectious conditions. Ultimately, this knowledge may accelerate the development of precision immunotherapies.
More information: Saeko Yanaka et al, Exploring glycoform-dependent dynamic modulations in human immunoglobulin G via computational and experimental approaches, Proceedings of the National Academy of Sciences (2025).
Journal information: Proceedings of the National Academy of Sciences
Provided by National Institutes of Natural Sciences