Weaving peptide nanonets to fight bacterial infections

Lisa Lock
scientific editor

Robert Egan
associate editor

Many harmful bacteria can move to evade high concentrations of antibiotics. This allows them to spread through the body and makes them harder to treat, contributing to the growing problem of drug-resistant infections.
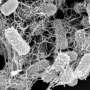
To tackle this, the research team led by Associate Professor Pui Lai Rachel Ee from the Department of Pharmacy and Pharmaceutical Sciences at NUS developed short peptides that can self-assemble into extremely fine fibers. These nanofibers weave together and form nanonets only in the presence of bacteria, trapping and killing them while leaving healthy cells untouched.
By immobilizing the bacteria, the nanonets prevent them from spreading and make them more vulnerable to existing antibiotics. The technology could inspire next-generation biomaterials for wound dressings, coatings for medical devices or spray-on treatments for bacterial infections.
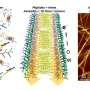
Drawing inspiration from spiders that first weave expansive webs to catch prey and then wrap them tightly to prevent escape, the team focused on designing peptides that can form both extensive and tight nets depending on their amino acid sequence. In this study, they discovered that subtle changes in the peptide's amino acids can control the way nanonets form.
The peptide W-W13 was engineered to form tightly interwoven nanofibers on bacteria surfaces, effectively trapping them in place and killing them. Another version, E-E13ASYM, was designed to self-interweave, forming wide-spanning nanonets with minimal bacterial interaction and no antibacterial activity.
The team showed through laboratory tests that the tight nanonets could significantly inhibit bacterial movement, highlighting their potential in preventing the spread of infection. The findings are in the journal Small.
Chen Wei Meng, a Ph.D. candidate on the research team, said, "Our findings show that with the right design, we can program these peptides to build nanonets tailored for specific antibacterial functions.
"By simply teaching a peptide how to fold, we have given it the ability to immobilize bacteria before they can escape. Our next step is to translate this into a practical, low-cost solution to combat infections in real-world settings," added Associate Professor Ee.
More information: Wei Meng Chen et al, Controlling Nanonet Morphology via Residue鈥怱pecific Modulation of 尾鈥怘airpin Peptide for Enhanced Bacterial Trapping, Small (2025).
Journal information: Small
Provided by National University of Singapore