RE1 proteins emerge as key players for amino acid transport in plants

Lisa Lock
scientific editor

Robert Egan
associate editor

Plants produce all the amino acids essential for human life. This commonly occurs in specialized cell organelles, so-called plastids. A research team headed by Heinrich Heine University Düsseldorf (HHU) has now decoded the mechanism by which plants distribute these amino acids within their organisms.
In an article in the journal Nature Plants, the researchers describe the mechanism and the class of transport proteins used for this process. The findings could potentially contribute to breeding crop plants with a higher content of essential amino acids and thus improving nutritional quality.
Proteins—the fundamental building blocks of every organism—are large molecules, which are made up of many so-called amino acids. Humans can produce some of these amino acids themselves, but others, the "essential amino acids," must be obtained from food. Plants synthesize all 20 "proteinogenic" amino acids from which proteins are comprised, making plants the ideal supplier of amino acids for the human diet.
However, plants do not produce the amino acids in all areas. Nine of these molecules, including important building blocks such as lysine and arginine, are only produced in the plastids. "Chloroplasts," in which photosynthesis takes place, are also plastids. Until now, it was unknown how the amino acids are transported from the plastids to other parts of the plant.
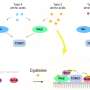
The research group headed by Professor Dr. Andreas P. M. Weber from the Institute of Plant Biochemistry at HHU has now attributed the function of transporting amino acids through chloroplast membranes to a class of transport proteins called RETICULATA1 (for short: RE1). This enables them to be exchanged within the plant.
Professor Weber, corresponding author, states, "The molecular function of RE1 has been a mystery for decades, even though it was known that mutations in this gene cause conspicuous leaf shapes in the model plant Arabidopsis thaliana (thale cress). We now show that RE1 is a specialized transporter for basic amino acids such as arginine, citrulline, ornithine and lysine."
Plants lacking RE1 not only have a characteristic "reticulated" leaf shape, but also only contain small amounts of basic amino acids in their leaves and chloroplasts. Lead author Dr. Franziska Kuhnert says, "This indicates a disrupted amino acid distribution in the plant. A complete loss of RE1 and its closest relative RER1 (RETICULATA-RELATED1) is even lethal to the plant, underscoring the essential role of these proteins."
The research team was also able to demonstrate that the loss of RE1 reduces the biosynthesis of basic amino acids and impairs the balance of amino acid pools between plastids and cytosol—the fluid within the cells.
Kuhnert states, "RE1 and related proteins are found exclusively in organisms that contain plastids. Since all plants and photosynthetic algae possess RE proteins, these proteins must be old in evolutionary terms and have originated from an era when plastids were formed through 'endosymbiosis'—the absorption of previously independent cells into other cells. RE1 may have made an important contribution to this evolutionary development of plants."
"Our results provide crucial insights into the complex connection between the transport of amino acids into plastids and leaf development, as well as nutrient distribution in plants," summarizes Weber. "The discovery opens up new perspectives for plant breeding and enables the development of crops with a higher content of essential amino acids. This can contribute to global food security."
More information: Franziska Kuhnert et al, RETICULATA1 is a plastid-localized basic amino acid transporter, Nature Plants (2025).
Journal information: Nature Plants
Provided by Heinrich-Heine University Duesseldorf