Study reveals simple peptides can mimic nature's protein protection strategy

Sadie Harley
scientific editor

Andrew Zinin
lead editor

A new study from researchers at the Advanced Science Research Center at the CUNY Graduate Center (CUNY ASRC) reveals that extremely simple peptides can mimic a biological process that protects sensitive proteins from environmental stress.
The findings, published in , offer a promising new approach to stabilizing biomolecules like vaccines and therapeutic proteins—potentially without the need for refrigeration.
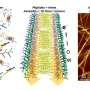
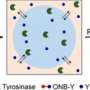
The study, led by Rein Ulijn, founding director of the CUNY ASRC Nanoscience Initiative and distinguished professor of chemistry at Hunter College, demonstrates how short peptides—just three amino acids long—can undergo liquid–liquid phase separation through a drying process that enables the peptides to encapsulate proteins, protect them, and release them intact upon rehydration.
"Inspired by how organisms like tardigrades survive extreme dehydration, we asked whether we could replicate nature's strategy using minimal synthetic materials," said Ulijn.
"To our surprise, we found that simple tripeptides could form dynamic, reversible structures that protect proteins under stress. This opens up new possibilities for protein preservation."
In biology, cells often respond to stress by creating protective compartments through a process known as phase separation. These compartments stabilize vulnerable proteins and can dissolve again when conditions improve.
The research team applied this principle to design adaptable peptide-based materials that mimic this process—offering a simple and effective alternative to conventional methods for biomolecular stabilization, which often require complex formulations and cold-chain logistics.
Key findings from the study include:
- Tripeptides can form reversible, disordered assemblies that undergo phase separation upon drying.
- These assemblies solidify into porous microparticles, efficiently encapsulating proteins.
- Upon rehydration, the peptides release their protein cargo with preserved structural integrity.
- The process mimics natural protective mechanisms and provides insight into a new mode of supramolecular material formation.
"This work not only reveals a novel mechanism of peptide self-organization but also introduces a minimalistic material platform for applications in biotechnology," said Ulijn.
The implications are far-reaching. From vaccine distribution in regions without reliable refrigeration to new classes of smart, responsive materials, the study lays foundational work for both practical innovations and further scientific exploration.
More information: Adaptive peptide dispersions enable drying-induced biomolecule encapsulation, Nature Materials (2025).
Journal information: Nature Materials
Provided by CUNY Advanced Science Research Center