How amyloid fibrils formed by RIPK1 and RIPK3 spread cell death between neighboring cells

Lisa Lock
scientific editor

Robert Egan
associate editor

In a study in the Proceedings of the National Academy of Sciences, researchers have revealed that amyloid fibrils formed by necroptosis mediators, RIPK1 and RIPK3, don't just form within dying cells, they can also escape the cell and trigger necroptotic death in neighboring cells.
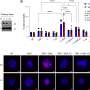
Using super-resolution microscopy, the researchers observed that as cells underwent necroptosis, RIPK1 and RIPK3 proteins co-assembled into fibrous, rod-like structures inside the cell. Within hours of necroptotic stimulus, these small protein puncta elongated into fibril-like assemblies in the cytoplasm, confirming that necroptosis involves an amyloid-like polymerization of its core signaling proteins.
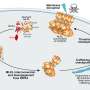
As necroptotic cells eventually ruptured, the internal RIPK1/RIPK3 fibrils were released into the surrounding medium. The researchers found that these extracellular fibrils could spontaneously enter nearby cells and seed the aggregation of the cells' own RIPK1 and RIPK3, thereby initiating the necroptosis program in previously unaffected cells.
In essence, the fibrils acted as transmissible "necroptotic seeds." Even fibrils reconstituted in vitro from the purified RIPK1 and RIPK3 RIP homotypic interaction motif (RHIM) domains were capable of inducing cell death when introduced into cells. This finding demonstrated that the amyloid form of these proteins is itself a functional signal capable of propagating cell death across cells.
To understand how one cell's death signal fibrils trigger another's, the researchers revealed the molecular structure of fibrils using cryo-electron microscopy. They found that RIPK1 and RIPK3 fibrils shared a nearly identical S-shaped β-sheet fold, which explained their cross-seeding ability. In other words, the two proteins' fibrils are structurally compatible, allowing a heterologous amyloid to form.
Furthermore, when the researchers introduced mutations that disrupted the S-shaped folding of the RIPK3 RHIM region, the mutant RIPK3 could no longer be recruited into fibrils by RIPK1 seeds, and the necroptotic cell death was effectively suppressed. This finding confirmed that the specific amyloid fold is critical for the intercellular transmission of the death signal.
This study reveals a previously unrecognized mode of cell-to-cell communication in programmed necrosis. Unlike the amyloid aggregates in Alzheimer's disease and Parkinson's disease, which pathologically spread neurodegeneration, the RIPK1/RIPK3 fibrils serve a functional signaling role in cell death. The necroptotic amyloids propagate an inflammatory form of cell death rather than a neurodegenerative pathology.
The researchers suggest that these necroptosis-related fibrils could even act as "seeds" that potentially initiate pathological amyloid formation in disease contexts. This study highlights an amyloid-based intercellular signal propagation mechanism in human cells, showing that cells can harness prion-like protein aggregation not just for causing disease, but as a deliberate means to amplify a death signal in certain biological scenarios.
The team was led by Prof. Yuan Junying and Prof. Liu Cong from the Shanghai Institute of Organic Chemistry of the Chinese Academy of Sciences.
More information: Yeyang Ma et al, Intercellular propagation of RIPK1/RIPK3 amyloid fibrils, Proceedings of the National Academy of Sciences (2025).
Journal information: Proceedings of the National Academy of Sciences
Provided by Chinese Academy of Sciences