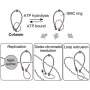
Cohesin is loaded onto the DNA double helix before replication (on the left). The replisome passes along the double helix, unzipping it and producing two identical sister copies. The team were keen to understand how the replication machinery navigates the cohesin ring, ensuring that both sister chromatids are entrapped after replication (on the right). Credit: Cell (2025). DOI: 10.1016/j.cell.2025.08.028
Before a cell divides, its DNA is replicated so that each daughter cell inherits the same genetic information. The two copies, known as "sister chromatids," are held together by a ring-shaped protein complex called cohesin until they are pulled to opposite poles of the cell in a miniature game of tug of war.
Cohesin was first discovered nearly 30 years ago, but scientists have long pondered how the DNA-copying machinery manages to navigate genetic strands while encountering cohesin on its path.
Crick group leaders John Diffley and Frank Uhlmann, were both working on different aspects of DNA metabolism, until new experimental methods in their respective fields brought them together to answer this question, in two research papers published today in and .
"We hadn't worked with cohesin at all; we had been busy investigating the elements that make up the DNA copying machinery called the replisome," says Diffley. "Meanwhile, Uhlmann and his team were making huge progress in understanding how cohesin keeps DNA organized and aids cell division. And it was fortuitous timing that we were both ready to explore how cohesin and the replisome interact at the same time."
The researchers recorded the interaction of cohesin and the replisome over time, which allowed them to see the replisome (in the center) pass through the cohesin ring (green) as the DNA is replicated (white strands). Credit: Samson Glaser, Cell.
Cohesin throws its hat into the ring
As part of his Ph.D., sitting across both Uhlmann and Diffley's labs, Samson Glaser led the experiments to recreate cohesin and replisome interactions.
"Hypothetically, the replisome should be able to fit through the ring, just based on their respective diameters," says Sam. "But even smaller DNA-bound obstacles are known to trip up these rings."
Sam used a method called "biological reconstitution," where the components of a process like DNA replication are mixed in a test tube. He added replisome enzymes to a piece of stretched-out DNA, which was loaded with cohesin, and fluorescently tagged all the components.
Looking down the microscope, Sam managed to see what had been, until now, only a hypothesis. "When we loaded cohesin onto DNA and added the replisome, in some cases we witnessed the replisome traveling through the ring," he says. "This suggests that cohesin is a malleable structure, allowing the replication machinery to pass."
"There was a bigger surprise, though," adds Diffley. "The more replisome components we added to the mix, the more efficiently the complex passed through cohesin rings, despite its increased size.
The researchers found that the components responsible were the enzymes that makes the new DNA strand, called DNA polymerases. When two of the main types of DNA polymerases were removed, the replisome passage was slower or couldn't happen at all.
Not the whole story
Sam's experiments also showed that slipping both DNA copies cleanly through the ring isn't always guaranteed.
His colleague Masashi Minamino, a postdoc working between the two labs, set out to investigate sister chromatid cohesion in more detail, revealing new insights into cohesin biology.
Graphical abstract. Credit: Molecular Cell (2025). DOI: 10.1016/j.molcel.2025.08.026
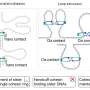
"Often, we saw that cohesin hugged just a single DNA copy after replication, making us think that the ring had ruptured when it met the replisome," says Minamino. "In our experiments, we observed that when this happens, a structure called the 'cohesin loader' appears to intervene and bring the second chromatid into the ring to join the first.
"We also showed that sometimes more cohesin molecules are used, bringing together the chromatids in a two-step process involving additional cohesin molecules to those present before replication."
"Copying DNA is one of the most important biological processes, so it makes sense that nature has more than one way of keeping the two replication products together," says Uhlmann.
Experiments using 2D gel electrophoresis showed how cohesin often ends up embracing only one of the two DNA replication products. The darker band on the bottom right is cohesin around just one replication product. Credit: Masashi Minamino, Molecular Cell.
Answering questions and asking new ones
For Diffley, these new insights satisfy some questions, but also open doors for future research. "We're now interested in how and why DNA polymerases are crucial for replisome passage through the ring in addition to their primary functions," he says.
And Uhlmann is keen to understand if the results in the lab mimic what's happening in the body. He says, "Now that we know there are many ways to keep genetic material together, we want to know if there's a preferred process or if certain ways are used in biological contexts. It feels like we're just getting started in this area."
He continues, "Without combining our expertise in the intricate components of DNA replication and how cohesin interacts with DNA, we wouldn't be able to answer these fundamental biological questions. This work could only have been done here at the Crick. It truly highlights the power of collaboration in science."
More information: Samson Glaser et al, Replisome passage through the cohesin ring, Cell (2025). .
Masashi Minamino et al, Biochemical reconstitution of sister chromatid cohesion establishment during DNA replication, Molecular Cell (2025). .
Journal information: Cell , Molecular Cell
Provided by The Francis Crick Institute