Electron microscopy reveals new method to make exotic metal alloys

Gaby Clark
scientific editor

Robert Egan
associate editor

Humans have been making metal alloys for thousands of years, and most of us can conjure a rough mental image of the process—it involves red-hot molten metals being mixed, poured, and shaped in a sweltering workshop or factory. This approach still works perfectly well for the traditional metals we see every day, like steel. But advanced metals with special chemical and mechanical properties, ones that scientists are investigating to use in energy technologies like long-lasting batteries and extreme-temperature engines for aerospace vehicles, need a more refined approach.
Researchers from the Department of Energy's Lawrence Berkeley National Laboratory (Berkeley Lab) have discovered a new way to produce these materials, called high-entropy alloys (HEAs), at near-room temperatures. Their technique, in a paper published today in Nature, also gives users much more control of the alloy's crystal structure and overall morphology compared with existing methods, opening the door for a new paradigm of custom-made HEAs.
First discovered about 20 years ago, these materials have generated a lot of excitement thanks to their record-breaking strength and toughness, giving them many potential applications in mechanical engineering. HEAs could also serve as potent catalysts, boosting the efficiency and durability of batteries and fuel cells and reducing our reliance on imported rare minerals. HEAs' unique properties stem from their balanced recipes of different elements.
While typical metal alloys are composed of a high proportion of one element with lower amounts of additional elements mixed in (for example, steel is ~97–99% iron with tiny amounts of carbon and other elements), HEAs are made of equal or nearly equal ratios of elements, creating an internal crystal structure with more entropy—meaning it is more disorganized.

And although this disorganization is key to their function, engineers still need to be able to tune the materials to certain specifications and form them into different shapes. The existing methods to make HEAs involve heating the elements to high temperatures so that the atoms have a lot of kinetic energy, mixing the different elements into one lump, then rapidly dropping the temperature by various methods. The drastic temperature change is thought to be necessary to lock in a state of internal disorganization from the energetic atoms.
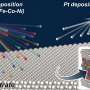
The Berkeley Lab team's approach achieves the same high-entropy result at much lower, constant temperatures (a breezy 25–80 degrees Celsius, or 77–186 F) by mixing the elements that will make up the HEA into the metal gallium when it is in liquid form. The elements are introduced in a water-based solution in their chloride forms. When the acidic liquid meets the liquid gallium at a pleasantly warm-to-hot temperature, the elements very quickly shed their chlorine atoms and mix together, then solidify into an HEA alloy.
According to team leader Haimei Zheng, a senior scientist in the Materials Science Division and adjunct professor at UC Berkeley, the incredible speed of the reaction and mixing at the liquid-liquid interface is what traps the much-desired entropy.
The new phenomenon was discovered by first author Qiubo Zhang, a postdoctoral researcher in Zheng's group, when he was conducting experiments with the team's liquid-cell transmission electron microscopy (TEM) platform, a technology they have advanced which allows scientists to study electronic and chemical reactions occurring in liquid environments in real time, at the atomic level. While using a liquid cell TEM to observe liquid gallium, he noticed Cu ions from the CuCl2 aqueous solution were getting sucked into the gallium and forming alloys.

"Because our new carbon film liquid cell has such high spatial and temporal resolution, we were able to observe the very quick transition at atomic level, just a tenth of a second, from a nanoscale amorphous liquid metal with the different elements to a small crystal," said Zhang. The finding inspired the team to try to create HEAs, which they were already interested in due to their work developing high-performance catalysts.
"It was a unique situation because other methods to make these alloys are hit and miss, and when it works, those scientists will come to researchers like us and use our TEM technology to understand the mechanism of how the alloy formed," said Zheng. "In this case, we discovered a mechanism with TEM first, then figured out how to use it for synthesis. We are very proud to have found another use for our technology."
Zhang's initial efforts to make HEAs with the process yielded nanoparticle-sized amounts. He and his colleagues then worked to scale the approach to make several grams of HEAs at a time. Their current technology, now patented, can generate HEAs in practical shapes other than round particles and with different types of crystal structure. They can also make HEAs with different composite metals, including ones without gallium, though that element is still key to the process.
Zheng's group is now collaborating with Kristin Persson, director of the multi-institutional Materials Project, Berkeley Lab faculty senior scientist, and Daniel M. Tellep, Distinguished Professor in Engineering at UC Berkeley, to accelerate the design of new HEAs using artificial intelligence. These breakthroughs will help move HEAs from promising experimental applications to real-world products.
The team is additionally exploring how they could use the technique to recover valuable rare minerals from wastewater generated by mining and geothermal wells. There are currently no cost-effective methods to pull these elements out of the water, meaning that domestic sources of commercially important metals like cobalt are going to waste. But a larger-scale version of the gallium-driven process has potential to selectively isolate these elements from the water and concentrate them in an alloy that could be further processed by customers, such as battery manufacturers.
More information: Qiubo Zhang et al, Isothermal solidification for high-entropy alloy synthesis, Nature (2025).
Journal information: Nature
Provided by Lawrence Berkeley National Laboratory