Flexibility is function: How unstructured protein segments regulate biological functions

Lisa Lock
scientific editor

Robert Egan
associate editor

Researchers have discovered how unstructured segments of surface proteins regulate the biological function of a cell. Their study, in Nature Communications, sheds new light on the interplay between G protein-coupled receptors (GPCRs)—located in the membranes of nearly all body cells—the messengers that activate them, and the signaling processes within a target cell. This interplay has a major role in drug discovery.
The joint team is from the Collaborative Research Center (CRC) 1423—Structural Dynamics of GPCR Activation and Signaling—at Leipzig University and Martin Luther University Halle-Wittenberg.
G protein-coupled receptors play a key role in many biological processes and are crucial for the effectiveness of medicines, for example, those used to treat high blood pressure, pain, allergies, or for weight reduction. Despite intensive research, many questions about the functioning of these important receptors remain unanswered.
The Y2 receptors examined in the study are activated by the peptide hormone neuropeptide Y (NPY), which influences various processes in the brain—such as stress responses and the circadian rhythm, but above all the transmission of satiety signals.
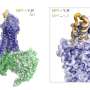
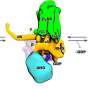
The researchers of CRC 1423 investigated the role of the flexible N-terminal segment of the Y2 receptor when the messenger NPY binds to the receptor and triggers a reaction in the cell. They found that this in particular affects the cellular partner protein arrestin-3—a key player that determines how the cell responds to a given signal.
Like many GPCRs, the Y2 receptor has a highly flexible, unstructured N-terminal region whose function is poorly understood. "What is special about this part of the receptor is that it is what is known as an intrinsically disordered region. It does not fold into stable structures, but instead constantly changes its shape.
"You can picture it as something like a cloud within which the protein segment moves. This happens so quickly that it is experimentally difficult to capture individual states and thereby assign specific functions to them," explains Dr. Anette Kaiser of Leipzig University and Professor Andrea Sinz of Martin Luther University Halle-Wittenberg, the study's lead authors.
To investigate these highly complex mechanisms, several research groups within CRC 1423 joined forces. Using a technique developed specifically for this receptor—a combination of light-induced cross-linking and highly sensitive mass spectrometry—the team led by Professor Andrea Sinz was able to pinpoint the exact sites of interaction between the Y2 receptor and NPY.
It became clear that the unstructured N-terminal region of the receptor comes into direct contact with the messenger NPY. This finding served as the starting point for extensive mutational studies on the receptor to examine the function of individual regions.
"We were able to see that short regions with a high density of negative charge are important for ensuring a stable, long-lasting binding of the peptide hormone to the receptor. Interestingly, some receptor-mediated cellular responses remain fully intact even when the contacts in the N-terminus are lost. However, interaction with the partner protein arrestin-3 is reduced by short-lived bindings, altering the balance of the cellular response," says Dr. Kaiser.
These observations were confirmed and further refined by the research groups of Professors Jens Meiler and Peter Hildebrand using computer-assisted structural modeling and molecular dynamics simulations. "Our understanding of the functional significance of flexible regions in signaling is still at a very early stage. This makes it all the more exciting to investigate whether the principles observed here can also be applied to other receptors," says Prof. Hildebrand of Leipzig University's Faculty of Medicine.
The working groups have been conducting joint research in this field for over four years, and these new findings build on extensive preliminary work. This makes this joint publication all the more valuable.
"Although there are still no approved medicines that act specifically via Y2 receptors, these findings provide further building blocks for gaining an even better understanding of the mechanism of action in the future and potentially identifying new targets for drugs," adds Professor Annette Beck-Sickinger, co-author and spokesperson of CRC 1423.
More information: Anette Kaiser et al, Transient ligand contacts of the intrinsically disordered N-terminus of neuropeptide Y2 receptor regulate arrestin-3 recruitment, Nature Communications (2025).
Journal information: Nature Communications
Provided by Leipzig University