Magnetic nanoparticles in synthetic cells enable controlled, deep-tissue drug release with reduced side effects

Sadie Harley
scientific editor

Robert Egan
associate editor

A synthetic cell that can be activated by a magnetic field to release a medicine while deep in the body has been created by chemists at UCL (University College London) and the University of Oxford.
The new technique, in Nature Chemistry, could be used to precisely target medicines for cancers or bacterial infections, simultaneously increasing their effectiveness and reducing side effects.
Synthetic cells are non-living mimics of real cells. They have a fatty membrane (or sac) that contains chemical or biological components such as DNA. Depending on the DNA inside, the cells can make any protein that is needed.
Their use for drug delivery has, until now, been limited by the fact they cannot be controlled once inside the body. Previous research activated synthetic cells with light, but light cannot penetrate more than a millimeter into the skin.
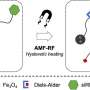
For these new synthetic cells, the research team attached DNA around tiny magnetic nanoparticles made of iron oxide. These DNA-coated nanoparticles were encased in a two-layer lipid membrane, just like the sac of a real cell.
The team applied an alternating magnetic field, causing the magnetic nanoparticles to heat up only their immediate surroundings, resulting in the activation of the DNA inside the synthetic cell to produce a protein. The DNA made a protein that glowed green, to measure how much was produced, and a membrane protein, for the controlled release of a model drug molecule.
For real-world applications, cancer- or bacteria-killing molecules can simply be swapped into this magnetic release system.
Being able to control when these molecules are released could allow these therapies to be used more precisely, potentially reducing side effects.
For this study, the researchers showed that the synthetic cells could be activated by a magnetic field even when they were inside a black tube, used to mimic the hard tissue of a living organism.
Senior author Dr. Michael Booth, based at UCL's Department of Chemistry, said, "What is exciting about our study is it opens up the potential for synthetic cells to be used in the body. It makes new kinds of treatments possible.

"Synthetic cells can be customized for a wide range of uses. They could in the future be engineered to release a medicine upon detecting something in their immediate environment—say, a tumor or bacteria. This more targeted approach could allow clinicians to use smaller doses of a treatment, making it safer.
"Our proof-of-principle work was carried out in water and the next step is for this technique to be tested with an anti-cancer 'cargo' targeting cancer cells in the lab."
The research team used "click chemistry," where molecules click together like LEGO bricks, to firmly bind the DNA to the surface of the magnetic nanoparticles, a technique used in some earlier studies.
However, as found in previous efforts, some DNA remained only loosely bound to the nanoparticles, meaning they came off too easily and leaked into the environment.
If used in living organisms, this is a problem as it could mean the cell's cargo (the proteins made by the heat-induced DNA activation) being released earlier than intended.
To combat this, the team developed a new method to pull off the loosely bound DNA strands. They embedded the nanoparticles in a gel and applied an electric field. As DNA is highly electrically charged, the loosely bound strands moved away from the particles, leaving in place only strands that were firmly attached.
This new technique reduced the amount of "leaky" DNA by 90%, the researchers calculated.
The researchers explained that both the heating and magnetic field strength were at levels safe for humans. This localized heating of a magnetic nanoparticle, triggered by an alternating magnetic field, is already used to treat glioblastomas (a type of brain cancer).
First author Ellen Parkes, a Ph.D. student at the University of Oxford, said, "Our proof of principle opens up the possibility of repurposing an already clinically approved therapy for killing cancer cells by utilizing alternating magnetic fields to make and release drugs only in the target area inside the body.
"The approach, using synthetic cells, is versatile and will enable different drugs that target a range of cancers to be made. This technology has the potential to become a new type of therapy with further research underway in a cancer model."
More information: Ellen Parkes et al, Magnetic activation of spherical nucleic acids enables the remote control of synthetic cells, Nature Chemistry (2025).
Journal information: Nature Chemistry
Provided by University College London