Scientists reveal the molecular choreography behind lanthanide separation in rare earth chemistry

Lisa Lock
scientific editor

Robert Egan
associate editor

What do magnets, smartphones and medical imaging devices have in common? They all depend on rare earth elements called lanthanides, which are vital for modern technology. Yet, separating these chemically similar elements from one another has long been one of chemistry's toughest puzzles.
Now, scientists at the U.S. Department of Energy's (DOE) Argonne National Laboratory have cracked open the mystery, revealing the molecular choreography that governs lanthanide separation—a breakthrough that could transform how we process these critical materials.
Lanthanides are a group of 15 metallic elements found near the very bottom of the periodic table. They are essential for technologies like magnets and are key components in catalysts—materials that speed up chemical reactions. However, they are chemically very similar and are often found together in ores, making them notoriously difficult to separate.
Michael Servis, an Argonne chemist, explained, "The DOE identifies lanthanides as critical materials due to their technological importance and potential supply risk. They are key targets for reprocessing and separations research."
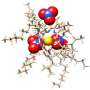
The team at Argonne used advanced computer simulations and experiments to reveal the hidden choreography of molecules during the extraction process. Traditionally, lanthanides are separated using a method called solvent extraction. In this process, the lanthanides are dissolved in an acidic solution and then selectively separated into an oil phase. Special molecules in the oil, called extractant molecules, bind to the lanthanides and help separate them.
Imagine a crowded dance floor where each dancer represents a molecule. The lanthanide ion is the star of the show, surrounded by extractant molecules, other ions and water molecules, all vying for the chance for a dance. The researchers found that the way these molecules "dance" around the lanthanide ion determines which element gets separated during extraction.
Using a simulation-based technique called metadynamics, the team created a map of the "energy landscape" of this molecular dance. This map shows the energy costs and benefits of different molecular arrangements.
Servis elaborated, "Metadynamics helps us see all the possible ways molecules can arrange themselves around the lanthanide. It's important to consider many possible arrangements, not just a single arrangement. This technique gives us clues about why some lanthanides are easier to separate than others."
The study found that lighter lanthanides, like lanthanum and europium, form stronger bonds with the extractant molecules. Heavier lanthanides, like lutetium, struggle due to crowding on the dance floor.
"The extractant molecule, ions and water must fit around the lanthanide, creating a crowded environment, which can affect extraction efficiency," Servis said.
The research also highlighted the role of water molecules in the dance. Some water molecules bind directly to the lanthanide ion and help stabilize interactions, forming hydrogen bonds that expand the possible dance moves. This variety is crucial for understanding the extraction trends and designing more efficient separation processes.
One surprising result was the unique trend in extraction selectivity across the lanthanides. Many conventional separation systems typically extract heavier lanthanides more easily, but this study observed the opposite.
"The trend is that lighter lanthanides are extracted similarly, but extraction efficiency decreases for heavier, more charge-dense lanthanides. Understanding this trend helps us design better systems for specific separation needs," Servis explained.
This discovery not only advances our understanding of lanthanide chemistry but also paves the way for more efficient and affordable ways to separate rare earth elements. Looking ahead, the team is exploring other solvents and extractant molecules that could improve selectivity even further.
Servis noted, "Our approach bridges fundamental coordination chemistry with real-world solution conditions, giving us insights that can make separation processes better."
The results of this research were in Chemical Science. Other contributors to this work include Xiaoyu Wang, Allison Peroutka, Dmytro Kravchuk and Richard Wilson from Argonne and Jenifer Shafer from the Colorado School of Mines.
More information: Xiaoyu Wang et al, Metadynamics investigation of lanthanide solvation free energy landscapes and insights into separations energetics, Chemical Science (2024).
Journal information: Chemical Science
Provided by Argonne National Laboratory