Ubiquitin's ability to tag synthetic compounds offers new path to drug discovery

Lisa Lock
scientific editor

Robert Egan
associate editor

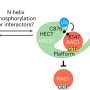
Small but powerful: Ubiquitin controls the lifespan and distribution of proteins in the cell, but it can also determine their shape, function, or interactions with other cellular components. Ubiquitin ligases are key to this process because they reliably recognize the relevant proteins among tens of thousands of molecules and confer the correct instructions. Disruptions of this precise tagging routine can result in faulty cellular processes and diseases such as cancer.
Conversely, drugs can be designed to modulate ubiquitin ligases, directing them to tag specific disease-promoting proteins with ubiquitin for degradation. However, this principle is currently only applicable to a few of the more than 600 human ubiquitin ligases.
The ubiquitin ligase HUWE1 is an interesting, yet unexploited target for therapeutic strategies with important roles in tumors and neurodevelopmental disorders. Although drug-like compounds have been used to inhibit HUWE1 in scientific research, their mechanism of action remained unclear, hindering their advancement into potential therapeutic agents.
Challenging discovery process
A team led by Sonja Lorenz at the MPI for Multidisciplinary Sciences has now unraveled the unexpected mechanism by which these compounds act. Using an interdisciplinary approach, the researchers combined protein biochemistry, cell biology, and click chemistry.
Other key methods for the project's success were tailor-made molecular syntheses from medicinal chemists led by Matthias Gehringer at the University of Tübingen, as well as the mass spectrometric measurements by Henning Urlaub's research group and molecular dynamics simulations by colleagues from Helmut Grubmüller's department at the MPI, Lorenz emphasizes.
Competition instead of inhibition
"We found that the drug-like compounds marketed as HUWE1 inhibitors indeed interact with this enzyme—but they do not inhibit it. Rather, HUWE1 recognizes the compounds themselves as target molecules and tags them with ubiquitin," reports Lorenz.
"When the drug-like compounds are supplied in excess over a natural target protein in the test tube, they consume the ubiquitin. This competition between the drug-like compounds and the target protein is what had previously been misinterpreted as HUWE1 inhibition," adds Pavel Pohl, one of the lead authors of the now published in the journal Nature Communications.
The team also succeeded in demonstrating, for the first time, that the synthetic compounds are tagged with ubiquitin in living cells. However, the situation here is much more complex than in the test tube.
"In the cell, HUWE1 promotes the ubiquitination of the compounds, but it does not drive it exclusively," explains Barbara Orth, another lead author of the study. This implies that cellular ubiquitination enzymes other than HUWE1 can also tag drug-like molecules with ubiquitin. This finding is of fundamental importance because ubiquitin signals had previously only been observed on proteins, sugars, and other biomolecules in cells, but not on synthetic compounds.
"The novel substrate spectrum will be particularly interesting for therapeutic and biotechnological applications in the ubiquitin field. Our discovery offers tangible strategies for developing new molecular tools to modulate the cellular ubiquitin system and, in turn, influence disease processes," explains Lorenz.
More information: Barbara Orth et al, Selective ubiquitination of drug-like small molecules by the ubiquitin ligase HUWE1, Nature Communications (2025).
Journal information: Nature Communications
Provided by Max Planck Society