Cryo-EM reveals how protein complexes maintain bacterial outer membrane defenses

Gaby Clark
scientific editor

Robert Egan
associate editor

Researchers from the National University of Singapore (NUS) have successfully applied cryo-electron microscopy (cryo-EM) to unveil the molecular structures of critical protein machines that transport lipids and maintain the outer membrane (OM) barrier of Gram-negative bacteria.
The rise in incidences of infections caused by antibiotic-resistant bacteria poses a global health threat. This is particularly worrisome with drug-resistant Gram-negative bacteria such as Escherichia coli (E. coli), which are already difficult to kill because their OM forms a formidable barrier against antibiotics.
To maintain the effectiveness of the OM in keeping drugs out, bacterial cells rely on various protein machines that work together to control the lipid composition and organization in the OM. However, the detailed mechanisms of these machines at the molecular level have remained elusive.
Research teams led by Associate Professor Chng Shu Sin at the Department of Chemistry, NUS, and the Singapore Center for Environmental Life Sciences Engineering (SCELSE-NUS), have made key discoveries on the inner workings of two such protein machines that transport lipids in the bacterial cell to maintain the OM barrier.
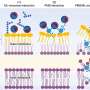
Applying single particle cryo-EM in combination with biochemical characterization, the teams solved the molecular details of the OmpC3-MlaA-MlaC and TolQ5R2A membrane protein complexes isolated from E. coli. They found that the OmpC3-MlaA complex deforms the local membrane, loosening misplaced lipids so they passage through MlaA and slip into the carrier protein MlaC.
Using two related structures of the TolQ5R2A protein complex, the teams also gained a better understanding of its role as a tiny, molecular motor to transmit force to help the cell maintain lipid balance in the OM.
These findings were published in the and .
Dr. Yeow Jiang, a senior research fellow in the Chng group and first author of both studies, said, "We are seeing, for the first time, the molecular architectures of the OmpC3-MlaA-MlaC and TolQ5R2A complexes, which opens up new possibilities for developing future antibiotics against these novel targets."
Gram-negative bacterial OM stability and function are critical for cellular survival and protection. By disrupting these protein machines that maintain the OM barrier, innovative therapeutic strategies can be devised to compromise bacterial membrane integrity, potentially overcoming the rising problem of antibiotic resistance.
"As a biochemistry research group, it is amazing that we can now learn and apply cryo-EM to gain molecular insights into membrane protein machines to elucidate their mechanisms in bacteria, which go a long way towards our fight against drug-resistant infections," added Prof Chng.
The team is now extending their research to study other bacterial lipid transport systems.
More information: Jiang Yeow et al, Structural Insights into the Force-Transducing Mechanism of a Motor–Stator Complex Important for Bacterial Outer Membrane Lipid Homeostasis, Journal of the American Chemical Society (2025).
Jiang Yeow et al, Molecular mechanism of phospholipid transport at the bacterial outer membrane interface, Nature Communications (2023).
Journal information: Journal of the American Chemical Society , Nature Communications
Provided by National University of Singapore