Discovery of brain parasite's unique control protein offers hope for better toxoplasmosis treatments

Stephanie Baum
scientific editor

Robert Egan
associate editor

Rajshekhar Gaji was staring at something that should not exist. Under his microscope, parasites that should have been thriving were instead dying—completely unable to survive without a protein his lab had managed to switch off.
"It was an amazing day," said Gaji, assistant professor of parasitology at the Virginia–Maryland College of Veterinary Medicine. That moment of discovery could eventually help the 40 million Americans walking around with a microscopic parasite permanently residing in their brains.
The findings are in the journal mSphere.
The invisible epidemic
Toxoplasma gondii lurks in the bodies of roughly one-third of all humans on Earth—about 40 million people in the United States alone, who carry this hidden passenger they'll never be able to eliminate.
Most never know it is there. For healthy individuals, the parasite typically causes no symptoms, remaining dormant for decades. But when the immune system weakens—during cancer treatment, organ transplantation, or HIV infection—this silent hitchhiker becomes a killer.
"The parasite that's sitting in the brain gets reactivated, starts multiplying, and then it's fatal," Gaji said. "Because of that, the parasite is a dreaded pathogen."
Pregnant women face a different danger. Toxoplasma can cross the placental barrier, causing miscarriages or severe congenital disabilities, including blindness and neurological damage.
Currently, there is no vaccine to prevent infection, and existing treatments only work during the acute phase of the disease—they cannot address the chronic form that establishes itself permanently in the brain.
Finding the master switch
Gaji's lab focuses on a family of proteins called transcription factors—molecular switches that control when genes turn on and off. Think of them as the parasite's mission control center, coordinating everything from invasion to survival.
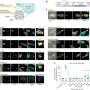
The team focused on one protein, TgAP2X-7, that appeared essential for parasite survival. Using a technique that involves plant hormone receptors, Gaji's lab engineered parasites in which this protein could be destroyed by adding a simple chemical.
When graduate student Padmaja Mandadi added that chemical, the results were dramatic.
"These parasites completely stop growing, and they cannot survive," Gaji said. "That shows this particular transcription factor is essential for the parasite to survive within the host."
The discovery revealed something more promising: This protein is fundamentally different from anything found in human cells. That uniqueness makes it an ideal target for future drugs—treatments could attack the parasite without harming patients.
Beyond one protein
But Gaji's research suggests TgAP2X-7 is just the tip of the iceberg. His lab has identified an entire family of understudied proteins called TKL kinases—eight different molecular switches that seem to orchestrate the parasite's most critical functions.
"Ours is the only lab currently studying this family of kinases in Toxoplasma," Gaji said. Six of these proteins are predicted to be essential for parasite growth, and his team has already shown that at least two control vital processes in the parasite's nucleus.
This represents a treasure trove of drug targets. While other researchers focus on obvious pathways, Gaji has carved out a unique territory in the parasite's control systems—the master switches that govern everything else.
A veterinary medicine breakthrough
For veterinarians and animal owners, this research carries special significance. Toxoplasma spreads primarily through cats, which serve as the definitive host for the parasite. The parasite completes its sexual life cycle in cats and is shed in their feces as oocysts, which then contaminate food, water, and soil.
"Cats act as the definitive hosts for this parasite," Gaji explained. Understanding how the parasite operates could lead to new prevention strategies protecting both animals and their human companions.
The work exemplifies how veterinary medicine increasingly drives innovation in human health. Gaji's background spans both animal and human pathogens, reflecting the interconnected nature of disease in our shared world.
His research approach—mapping the fundamental control systems that parasites use to survive—could prove valuable beyond the context of toxoplasmosis. Many disease-causing parasites rely on similar molecular machinery, suggesting insights from this work might translate to treatments for malaria, cryptosporidiosis, and other apicomplexan diseases.
The long road to treatment
Developing a new drug typically takes a decade or more, but Gaji's work provides a crucial foundation for that journey. The financial incentives are substantial: with 30% of the global population infected, effective treatments for toxoplasmosis represent a significant market opportunity.
"It is a significant parasite infection that needs a lot of research and financial investment," Gaji said.
Gaji's lab is now working to understand precisely how TgAP2X-7 controls parasite survival. His lab has identified the specific DNA sequence this protein recognizes—essentially cracking part of the parasite's genetic code.
The next step involves mapping which genes this master switch controls and understanding why its disappearance proves fatal.
"We want to understand why these different amino acids in this protein are actually involved in binding to that particular motif in gene promoters," Gaji said.
The research also opens new frontiers in parasite biology. By studying how these master switches operate during different stages of the parasite's complex life cycle, scientists might find vulnerabilities that could be exploited therapeutically.
As Gaji continues mapping the parasite's control systems, he is building toward something bigger than treating a single disease. Gaji is developing a new understanding of how some of humanity's most persistent microbial enemies operate—knowledge that could reshape how we fight infectious diseases for generations to come.
The parasite that has quietly infected billions may have just met its match in a lab in Blacksburg, where researchers are learning to speak its molecular language and turn its own control systems against it.
More information: Padmaja Mandadi et al, TgAP2X-7 is a novel cell cycle-regulated transcription factor that plays an essential role in Toxoplasmatachyzoite propagation, mSphere (2025).
Provided by Virginia Tech