Splitting water: How order and disorder direct chemical reactivity

Stephanie Baum
scientific editor

Robert Egan
associate editor

In nature, the behavior of systems—whether large or small—is always governed by a few fundamental principles. For instance, objects fall downward because it minimizes their energy. At the same time, order and disorder are key variables that also shape physical processes. Systems—especially our homes—tend to become increasingly disordered over time. Even at the microscopic level, systems tend to favor increased disorder, a phenomenon known as an increase in so-called entropy.
These two variables—energy and entropy—play an important role in chemical processes. Processes occur automatically when energy can be reduced or entropy (disorder) increases.
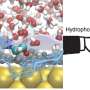
Under standard conditions—such as in a glass of water—water autodissociation is hindered by both factors, making it a highly unlikely event. However, when strong electric fields are applied, the process can be dramatically accelerated.
Now, researchers at the Max Planck Institute for Polymer Research and the Yusuf Hamied Department of Chemistry at the University of Cambridge have uncovered a surprising mechanism that governs water autodissociation in such intense fields.
Their findings, in the Journal of the American Chemical Society, challenge the traditional view that this reaction is mainly driven by energy considerations.
"Water autodissociation has been extensively studied in bulk conditions, where it's understood to be energetically uphill and entropically hindered," says Yair Litman, group leader at the Max Planck Institute. "But under the strong electric fields typical of electrochemical environments, the reaction behaves very differently."
Using advanced molecular dynamics simulations, Litman and co-author Angelos Michaelides show that strong fields dramatically enhance water dissociation—not by making the reaction more energetically favorable, but by making it entropically favorable. The electric field initially orders water molecules into a highly structured network. When ions form, they disrupt this order, increasing the system's entropy—or disorder—which ultimately drives the reaction forward.
"It's a complete reversal of what happens at zero field," explains Litman. "Instead of entropy resisting the reaction, it now promotes it."
The study also shows that under strong electric fields, the pH of water can drop from neutral (7) to highly acidic levels (as low as 3), with implications for how we understand and design electrochemical systems.
"These results point to a new paradigm," says Michaelides. "To understand and improve water-splitting devices, we need to consider not just energy, but entropy—and how electric fields reshape the molecular landscape of water."
The research highlights the need to rethink how reactivity is modeled in aqueous environments under bias and opens up new possibilities for catalyst design, particularly in electrochemical and "on-water" reactions.
More information: Yair Litman et al, Entropy Governs the Structure and Reactivity of Water Dissociation Under Electric Fields, Journal of the American Chemical Society (2025).
Journal information: Journal of the American Chemical Society
Provided by Max Planck Society