Developing drugs—with tens of thousands of minuscule droplets on a small glass plate

Sadie Harley
scientific editor

Robert Egan
associate editor

A glass plate, a delicate tube and an oil bath are all that is required: thanks to a new method, researchers at ETH Zurich can produce tens of thousands of tiny droplets within minutes. This enables them to test enzymes and active ingredients faster, more precisely and in a more resource-efficient manner than previously.
What happens when an enzyme encounters a potential active ingredient that is supposed to inhibit or activate the enzyme? This is precisely what drug development is all about. Analyzing the interaction of an enzyme with an active ingredient molecule, however, is extremely complex.
The group led by Petra Dittrich, Professor of Bioanalytics at ETH Zurich, has developed a that radically simplifies such tests: their method allows up to 100,000 minuscule droplets containing enzymes and substrates to be produced on a glass plate—in a mere 40 minutes and without involving a pipette.
The work is published in the journal Small.
To date, most researchers have used microtiter plates for such analyses, which are hand-sized standardized plastic plates with up to around 1,500 small wells. Each of these recesses is essentially a mini test tube that is filled with pipettes. The new method developed by the ETH researchers is far more efficient, resource-friendly and flexible.
Droplets landing with pinpoint accuracy
At the heart of the method is a coated glass plate that is about the size of a microscope slide, measuring around two by seven centimeters. Its surface is covered with a water-repellent layer that has been exposed at up to 100,000 points: tiny water-loving (hydrophilic) landing sites for droplets. The test solution is fed through a delicate tube onto the glass plate, which moves under precise control.
As soon as the liquid reaches a hydrophilic point, a tiny drop separates and adheres precisely there. The droplets are tiny: depending on the size of the glass plate, their diameter ranges from 50 to 250 micrometers—thinner than a human hair and barely visible to the naked eye.
In order to keep the droplets stable, the entire glass plate is submerged in a shallow oil bath. The oil prevents the tiny amounts of liquid from evaporating, even during day-long experiments, while the oil protects against contamination. After the experiment, the oil can even be collected, cleaned and reused.
The entire droplet production process is automated by way of a compact device the size of a microscope that can also perform microscopy, cultivate cells and automatically change samples.
"It used to take half an hour to set everything up correctly," Breitfeld relates. "Today, thanks to our automation, all it takes is the push of a button and the experiment is up and running," says Maximilian Breitfeld, a scientist in Dittrich's research group.
What's more, the combination of precise control and physical oil protection allows the composition of the droplets to be varied in a targeted manner—sometimes with more, sometimes with less active ingredients, resulting in fine concentration gradients.
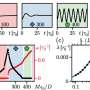
Researchers can make use of this to observe enzymes over a longer period of time, for example, or to investigate the effect of active ingredients on cells in highly parallelized tests. The droplets are then analyzed either by fluorescence microscopy or mass spectrometry, allowing enzyme reactions to be tracked precisely.
When hours become minutes
The basic idea stems from earlier work performed by the group in which they had already generated droplets for screening applications.
"We knew how the technology worked in principle, but it was too slow to be competitive in practice," says Dittrich. The former doctoral students, Maximilian Breitfeld and Claudius Dietsche, took the decisive steps: their newly developed process not only massively accelerates drop generation but also automates the entire process.
ETH Zurich has patented the process and nominated it as a finalist for this year's Spark Award.
Managing a huge amount of data
The method's tremendous potential also entails a new challenge. "We generate a huge amount of data," says Dittrich. "It's no longer possible to evaluate this manually—we need software solutions that help us analyze the information in a meaningful way."
While the amount of data is rapidly on the rise, resource consumption remains surprisingly low: up to five kilograms of non-recyclable plastic can be saved per experiment, the oil used can be collected, while chemical consumption is drastically reduced compared to conventional methods, given that an entire experimental run requires only microliters instead of liters of reaction medium.
Nevertheless, the method does have clear limitations. Whereas the tiny droplets are perfect for rapid reactions in small volumes, as is common in microfluidics, the new method is not suitable for larger volumes of liquid or tissue cultures that grow over several weeks.
From research to spin-off
The researchers are now planning a spin-off to launch the method on the market. They plan to sell a complete system consisting of glass plates, equipment and software—and biological tests based on it as an option.
"For me, it is crucial that the system is genuinely reliable and easy to use," says Dietsche. "It can only be used beyond our research laboratory if we can guarantee its user-friendliness."
Demand is already evident, the researchers report, and the nomination for the Spark Award is lending them additional momentum for the planned spin-off.
More information: M. Breitfeld et al, Ultrafast Formation of Microdroplet Arrays with Chemical Gradients for Label‐Free Determination of Enzymatic Reaction Kinetics, Small (2025).
Journal information: Small
Provided by ETH Zurich