Enzyme-based system produces versatile active ingredients for drug discovery and testing

Lisa Lock
scientific editor

Robert Egan
associate editor

Natural products derived from microorganisms are a promising source of new active ingredients, but are often produced only in very small quantities. A research team from the Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) led by Tobias Gulder has now succeeded in establishing a chemo-enzymatic platform for the production of furanolides—a class of natural products with a broad spectrum of activity.
This strategy not only allows furanolides to be produced cost-effectively, but also enables structural modifications to be made. The newly generated molecules are capable of effectively combating both bacterial pathogens and cancer cells. The team has now its findings in the Journal of the American Chemical Society.
Furanolides are a class of natural products with high structural diversity and a broad spectrum of biological activities. While some members of this group are very effective against bacteria, others are capable of killing algae or even human cells. Furanolides thus offer a promising basis for the development of new drugs.
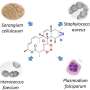
In addition to cyanobacteria and myxobacteria, natural sources of furanolides include some marine animals such as ascidians. What they all have in common is that they produce furanolides only in very small quantities, which has made them difficult to study in detail in terms of their structure and activity.
With the help of a newly developed chemo-enzymatic platform, a research team led by HIPS department heads Gulder and Rolf Müller has now succeeded in producing a large number of different furanolides in larger quantities and characterizing their biological activity. HIPS is a site of the Helmholtz Center for Infection Research (HZI) in collaboration with Saarland University.
In addition to the production of furanolides by microorganisms, it is also possible to produce them entirely by chemical synthesis. However, the synthesis routes known to date suffer from low yields and are very cost-intensive. To circumvent these problems, Gulder and Müller have developed an approach that uses individual enzymes from furanolide biosynthesis to assemble the natural products in a test tube.
The knowledge about the relevant enzymes comes from a study conducted by Gulder's team in 2022, in which the researchers successfully uncovered the biosynthesis of precyanobacterin, a member of the furanolide family.
The newly developed strategy uses the two previously discovered enzymes CybE and CybF to assemble the furanolide backbone from a series of simple precursor molecules. In order to be able to produce previously unknown representatives of furanolides in addition to precyanobacterin, the team compiled a series of modified precursor molecules and tested whether these could also be converted by CybE and CybF.
"We were able to identify dozens of different precursor molecules that can be converted efficiently enough by our CybE/F system. By combining these substrates in different ways, we were able to generate a substance library with a total of 385, mostly new, furanolide derivatives," says Gulder, head of the department Natural Product Biotechnology.
"Subsequently, by optimizing the supply of precursor substances, we were able to significantly reduce the costs of furanolide production in our system. This enabled us to produce individual derivatives in the quantities we need to test their biological properties."
Based on the structural properties of the new furanolides, the team selected 17 of the 385 possible derivatives to characterize their biological activity against bacterial pathogens and cancer cells in more detail. Jennifer Herrmann, senior scientist in Müller's department, says, "All of the furanolides tested were able to kill human cancer cells in the laboratory—some of them even more effectively than drugs already in clinical use. We observed that our substances could even eliminate cancer stem cells effectively.
"In addition, some furanolide derivatives are capable of inhibiting the growth of several Gram-positive pathogens such as Staphylococcus aureus. The substance class thus offers numerous possibilities for future developments."
The team is currently using the knowledge gained about the relationships between the structure and activity of furanolides to further optimize selected derivatives. In the long term, the aim is to determine whether the substance class is suitable for the development of active substances for the treatment of infectious diseases or cancer.
More information: Xiaoqi Ji et al, A Chemo-Enzymatic Platform for Furanolide Synthesis and Functional Exploration, Journal of the American Chemical Society (2025).
Journal information: Journal of the American Chemical Society
Provided by Helmholtz Association of German Research Centres