Structural surprise in motor protein may point to new strategies for controlling disease

Stephanie Baum
scientific editor

Robert Egan
associate editor

Motor proteins are tiny "machines" inside cells that use chemical energy to move along molecular tracks and carry out essential processes like chromosome segregation during cell division. When a cell splits to make two new cells (called daughter cells), it carefully shares its instructions (chromosomes) so each new cell knows how to grow and work properly.
A group of motor proteins known as kinesin-8 proteins helps regulate how chromosomes are distributed between daughter cells—a process that—when disrupted—can lead to genomic instability. This instability is a key factor in the development of many diseases, including cancer.
"You can think of kinesins as tiny robots walking along train tracks to help organize and move chromosomes during cell division," says John Allingham, professor and associate head of research in the Department of Biomedical and Molecular Sciences at Queen's University.
While most research on kinesins has focused on the "feet" or motor domains—regions that walk along microtubule tracks—Allingham's group turned their attention to the less-studied "body" or stalk region, which connects the feet and enables them to work together.
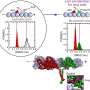
Recently, Allingham and his colleagues determined the structure of the stalk region of the fungal kinesin-8 protein Kip3, using Canada's only synchrotron research facility, the Canadian Light Source (CLS) at the University of Saskatchewan. Their findings, in Structure, reveal an unexpected architecture that could reshape our understanding of how kinesin-8 proteins assemble and function.
"What we expected to find was a long, coiled structure typical of other kinesin families," says Allingham. "Instead, we discovered that this region folds into a compact helical bundle—more like a folded camping chair than a long, flexible pole."
The team believes this compact bundle structure plays a key role in how kinesin-8 proteins join into pairs (dimerize) and move along a cell's microtubule tracks. Because kinesin-8 proteins are essential for proper chromosome segregation, understanding their architecture could help explain mechanisms of chromosomal instability, a hallmark of many cancers, and identify unique structural features in fungal kinesins that might be exploited for antifungal drug design.
"Our work doesn't target cancer directly," notes Allingham. "But by studying how fungal kinesin-8s assemble and function, we can uncover principles that apply broadly to cell division and identify new ways to inhibit proliferation of disease-causing cells—an area of intense medical concern."
Allingham emphasizes that discoveries like this rely on advanced tools such as the CLS.
"Using the synchrotron to see these protein structures at high resolution lets us uncover surprises that you'd never predict," he says. "Even after decades of studying these motors, nature still has tricks up its sleeve."
More information: Daria Trofimova et al, Fungal kinesin-8 motors dimerize by folding their proximal tail domain into a compact helical bundle, Structure (2025).
Journal information: Structure
Provided by Canadian Light Source