Credit: Journal of the American Chemical Society (2025). DOI: 10.1021/jacs.5c07355
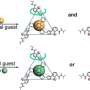
Researchers have engineered a novel M6L4 octahedral molecular cage by integrating photoactive cyclometalated platinum(II) units, creating a visible-light-responsive "nanoreactor" that drives highly efficient photochemical reactions through precise molecular confinement. This innovative design overcomes the limitations of previous hosts, achieving perfect stereo- and site-selective cross-[2 + 2] cycloaddition reactions, and most notably, enabling catalytic cross-[2 + 2] cycloaddition of chemically inert substrates using this supramolecular confinement approach.
The research is in the Journal of the American Chemical Society.
Supramolecular coordination cages function as versatile nanoreactors, leveraging confined space to preorganize reactants and significantly enhance the selectivity of chemical transformations, known as host-guest complexation. Photochemical reactions are highly valued in synthetic chemistry for enabling difficult chemical transformations under mild, energy-efficient conditions.
However, previous highly effective M6L4 molecular cages typically lacked visible-light absorption, restricting their utility to substrates requiring UV light or external energy transfer, which limited the range of possible reactions. This new study aimed to overcome this fundamental limitation by constructing a cage that combines robust molecular recognition capabilities with intrinsic visible-light sensitivity to unlock its full potential for photosensitized transformations.
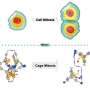
The team synthesized a new M6L4 octahedral cage (Cage 2) by systematically replacing the photoinert metal units at every vertex with visible-light-active cyclometalated platinum(II) complexes. This modification allowed the cage itself to absorb visible light (up to 430 nm) via a Metal-to-Ligand Charge Transfer (MLCT) transition while retaining the excellent guest-binding ability of standard cages. They tested the cage's capacity to facilitate cross-[2 + 2] cycloaddition reactions using visible light (465 nm LED) between maleimides and various photoinactive unsaturated compounds, including pyrenes and electron-deficient olefins like cinnamic acid.
The engineered Cage 2 successfully promoted highly efficient cross-[2 + 2] cycloadditions for numerous inert substrates, consistently demonstrating perfect stereo- and site-selectivity—a direct consequence of the precise preorganization achieved through molecular confinement. The reaction mechanism relies on photoinduced Energy Transfer (EnT) from the excited cage to the confined substrate, supported by the finding that the cage's triplet excited state (SOMO) is delocalized onto the triazine panel ligand, accelerating energy transfer through orbital overlap with the guest.
Most significantly, the system achieved the first catalytic cross-[2 + 2] cycloaddition between N-ethylmaleimide and cinnamic acid, resulting in the trans-syn stereoisomer with a high yield (78%) and a Turnover Number (TON) of 5.2, achieved because the non-bulky product does not bind tightly, allowing efficient guest exchange and sustained catalysis.
This research introduces a powerful, mechanism-based strategy for integrating visible-light-responsive functionality into supramolecular coordination cages through targeted capping ligand design, offering a major advantage over conventional photocatalysts lacking effective molecular recognition sites. The success in catalyzing the cross-[2 + 2] cycloaddition between two electron-deficient substrates (maleimide and cinnamic acid) is particularly rare and highlights the utility of this cage system for complex and challenging transformations.
By achieving high efficiency and perfect stereocontrol using visible light, this study significantly expands the potential scope of photo-organic chemistry, paving the way for the development of new self-sensitizing nanoreactors for green and selective synthesis.
More information: Rikuya Tanaka et al, A Visible-Light-Responsive Octahedral Cage for Efficient and Selective Cross-[2 + 2] Cycloadditions, Journal of the American Chemical Society (2025).
Journal information: Journal of the American Chemical Society
Provided by National Institutes of Natural Sciences