Dancing proteins keep cells moving: Redefining the function and role of key factors in actin filament disassembly

Sadie Harley
scientific editor

Robert Egan
associate editor

Some cells, such as immune cells, are highly mobile—they constantly remodel their shape, migrate toward a wound that needs to be closed or chase down bacteria in the bloodstream. This mobility is provided by the cytoskeleton, a complex network of filaments continuously being assembled and dismantled.
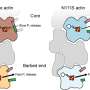
How the disassembly of actin filament is orchestrated by the key factors coronin, cofilin, and AIP1 has now been solved by a team led by Stefan Raunser at the Max Planck Institute of Molecular Â鶹ÒùÔºiology in Dortmund (MPI).
Their study, published in , redefines the roles of these proteins and provides molecular details that improve our understanding of how both healthy and malignant cells move through the body.
Cells grow, change shape, move, and divide. They give structure to tissues, close wounds, and hunt down bacteria in the blood. This mobility is a prerequisite for a variety of essential biological functions such as immunity, but it also underlies pathological events like metastasis.
The cell's mechanical stability and its ability to move are ensured by the cytoskeleton—a dynamic network of protein tubes and filaments. Actin filaments play a major role in this system. They self-assemble by polymerizing individual actin proteins.
Need for speed
"On average, cells can travel approximately 30–50 micrometers per hour—roughly 1 mm per day. For a micrometer-sized cell, that is certainly not a fast pace," says Stefan Raunser, Director at the MPI Dortmund. "The molecular process underlying the movement, however, must occur at 'breakneck' speed."
Within seconds, actin filaments grow underneath the cell membrane pushing it forward. Almost as quickly, those filaments must be disassembled to prevent unproductive elongation and to ensure optimal power transmission to the membrane. The disassembly is regulated by a trio of proteins—coronin, cofilin and AIP1—but the underlying mechanisms had remained elusive so far.

Squeezing out every bit of speed
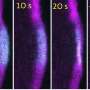
"Using cryo-electron microscopy, we obtained 16 3D structures that show how these proteins act together on actin filaments," explains Wout Oosterheert, first author of the study and former Postdoc in the Raunser laboratory (now junior group leader at the Netherlands Cancer Institute).
"For the very first time, we could visualize actin filament disassembly in this high detail, and the process turned out to involve several coordinated steps. In other words, we uncovered a dance between proteins—a molecular choreography."
First, coronin sticks to the filament and allosterically accelerates the release of the phosphate that remains bound to actin after ATP hydrolysis. This also triggers a small change in the twist of the filament, which primes the filament for binding by multiple cofilin proteins.
Cofilin binding pushes coronin off the filament to create a binding platform for AIP1, which then acts like a clamp: It grabs and "squeezes" the filament, breaking down the connections between the actin units, ultimately causing rapid severing.
From structure to therapy?
Many steps of the elucidated mechanism had not been anticipated before. Previous research by other groups had suggested that cofilin was the main protein that severs the actin filament, with AIP1 acting only as a helper protein. However, the study by the Max Planck researchers demonstrates that AIP1 is the actual protein that performs the severing.
"Our structural study enabled us to redefine the roles of the key factors in actin filament disassembly," says Raunser. Dysregulation of any of these proteins is linked to a wide range of diseases—from cancer to immune disorders to myopathies.
"Our work now provides a mechanistic framework for actin dynamics which may ultimately contribute to the development of new therapeutic agents," adds Oosterheert.
"From a scientific perspective, it is also simply exciting that we could visualize the synergistic actions of coronin, cofilin, and AIP1 in such detail. It highlights how tightly regulated actin network disassembly actually is."
More information: Wout Oosterheert et al, Choreography of rapid actin filament disassembly by coronin, cofilin, and AIP1, Cell (2025).
Journal information: Cell
Provided by Max Planck Society