Scientists discover regulatory pathway behind cyanobacteria's carbon-fixing factories

Gaby Clark
scientific editor

Robert Egan
associate editor

Long before plants and algae, cyanobacteria were already performing photosynthesis‚ÄĒfilling Earth's skies with oxygen and setting the stage for life as we know it. The ultra-prevalent bacteria are critical to the global carbon cycle, responsible for fixing as much as 30% of the world's carbon dioxide and converting it into oxygen we breathe. And because they're the simplest organisms that carry out this complex process, they're both ideal for studying fundamentals of biology as well as promising contenders for bioengineering.
Danny Ducat's lab at Michigan State University is working to unravel how cyanobacteria manage this carbon fixation process. Ducat is professor of Biochemistry and Molecular Biology and the MSU-Department of Energy Plant Research Laboratory.
In a new study in The Plant Journal, a team led by postdoctoral researcher Mar√≠a Santos-Merino illuminates a key regulatory pathway between cyanobacteria's light-harvesting systems and the inner compartments where carbon fixation happens. It's an important step toward better understanding how cyanobacteria balance their energy demands‚ÄĒand how their productivity might be ramped up to support better biotechnologies.
Carboxysome control
Photosynthesis in cyanobacteria is different from that in plant cells, and researchers are still uncovering how their inner machinery operates.
"It's a really interesting organism, not only because they are able to grow with light and minimal nutrients, but also because we don't know a lot about them compared to other bacteria, like E. coli," Santos-Merino said.
Previous work in the Ducat lab found that when cyanobacteria were engineered to secrete sugar, they also became more efficient at photosynthesis. This was surprising, Ducat said, as microbes engineered in this way typically lose, rather than gain, productivity.
He called it a fortuitous discovery because it represented a win-win situation‚ÄĒwhen the organisms are engineered to make something valuable, they also get better at their primary role of CO2 fixation. When the team began to investigate why, they traced the effect to a protein called RpaA (Regulator of Phycobilisome Association A), but its role wasn't clear.
Santos-Merino, who has long been fascinated by RpaA, explains that the protein was once considered to only be part of cyanobacteria's circadian clock, serving as a sort of master switch controlling rhythms of gene expression. But more recently, researchers have found the protein has even more roles.
"We don't know the full extent of the function of RpaA right now," she said. "It's remarkable to find new functions of this important protein."
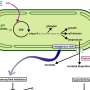
Inside each cyanobacterium are compartments called carboxysomes‚ÄĒprotein shells that house Rubisco, the enzyme that captures carbon dioxide. In the new study, Santos-Merino found that RpaA functions at the interface between the cell's light-gathering machinery and its carboxysomes, serving as a messenger to help the compartments grow or shrink according to changing energy supplies.
To reveal the full pathway at work, the researchers created mutant strains of cyanobacteria lacking the RpaA protein and compared them to normal cells. They used fluorescent tags to watch carboxysomes under a microscope, exposed the microbes to different environmental conditions such as changes in light and carbon supply, and measured how much CO‚āā and O‚āā the cells consumed or released. Together, these approaches let the team see the structural changes inside the cells and their effects on photosynthesis.
The team found that without RpaA, carboxysomes disintegrate completely when their cyanobacterium is under stress.
"That was a bit of a surprise, because they're usually there all the time," Ducat said. "They're required for growth of the cyanobacteria."
The breakdown could be reversed when the stress was removed, suggesting a dynamic regulatory system.
A foundation toward improving photosynthesis
This foundational science is exactly the kind of discovery that underpins future innovation. Because cyanobacteria are so efficient at turning sunlight into sugars, researchers envision them fueling systems that produce a wide range of biomaterials, without the need for arable soil or potable water.
"To explore the full biotechnological potential of cyanobacteria, we need to understand every detail of the processes inside them," Santos-Merino said. "We still have a lot of things to discover."
More information: Mar√≠a Santos‚ÄźMerino et al, Plastoquinone redox status influences carboxysome integrity via a RpaA‚Äź and reactive oxygen species‚Äźdependent regulatory network, The Plant Journal (2025).
Journal information: The Plant Journal
Provided by Michigan State University