Watching Proteins Direct Crystal Growth One Step at a Time (w/ Video)
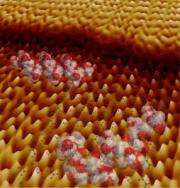
(�鶹��ԺOrg.com) -- Scientists at Berkeley Lab's Molecular Foundry imaged the growth of protein-studded mineral surfaces with unprecedented resolution and provided a glimpse into how living systems engineer key structural materials.
Scientists at Lawrence Berkeley National Laboratory’s Molecular Foundry have imaged the growth of protein-studded mineral surfaces with unprecedented resolution, providing a glimpse into key structural materials engineered by living systems. The team’s high-resolution technique reveals the natural mechanisms employed by creatures at sea and on shore alike, and could provide a means to observe and steer this crystal growth as it occurs.
For millions of years, organisms from algae to humans have used biomineralization-the process of organizing minerals such as calcium carbonate into biological systems-to generate shells, spines, bones and other structural materials. Recently, researchers have begun to unravel the structure and composition of these biominerals. However, understanding how biomolecules interact with minerals to form these complex architectures remains a formidable challenge, as it requires molecular-level resolution and rapid-imaging capabilities that don’t disturb or alter the local environment.
Video: Atomic force microscopy movie shows a peptide adsorbed to a crystal surface while two successive crystal steps interact, then progress beyond the peptide. The peptide temporarily slows the step before transferring up to the next atomic layer. The lattice pattern on the surface corresponds to the molecular structure of the underlying crystal.
Atomic-force microscopy, which tracks nanometer-scale hills and valleys across a crystal’s terrain with a sharp probe, is often used to study surfaces. The deflections a probe encounters across a material are translated into electrical signals then used to create an image of the surface. However, a careful balancing act is required to maintain the resolution provided by a sharp probe and the flexibility needed to leave soft biological molecules unperturbed. Now, Molecular Foundry researchers have developed a tool able to discern delicate biological materials and minute undulations on a crystal’s surface-all while watching the mineralization process in the presence of proteins.
“We’ve found an approach to consistently image soft macromolecules on a hard crystal surface with molecular resolution, and we’ve done it in solution and at room temperature, which is much more applicable to natural environments,” says Jim DeYoreo, deputy director of the Molecular Foundry, a U.S. Department of Energy National User Facility located at Berkeley Lab that provides support to nanoscience researchers around the world.
“With these hybrid probes, we can literally watch bio-molecules interact with a crystal surface as the crystal grows one atomic step at a time. Nobody has been able to watch this process with this kind of resolution until now,” says Raymond Friddle, a post-doctoral scholar at Lawrence Berkeley National Laboratory.
DeYoreo, Friddle, co-authors Matt Weaver and Roger Qiu (Lawrence Livermore National Laboratory), Bill Casey (University of California, Davis) and Andrzej Wierzbicki (University of Southern Alabama), used these ‘hybrid’ atomic-force microscope probes to study the interactions between a growing crystal of calcium oxalate monohydrate, a mineral present in human kidney stones, and peptides, polymer molecules that carry out metabolic functions in living cells. These hybrid probes combine sharpness and flexibility, which is crucial in achieving the speed and resolution required to monitor the growing crystal with minimal disturbance to the peptides.
The team’s findings reveal a complex process. On a positively charged facet of calcium oxalate monohydrate, peptides form a film that acts like a switch to turn crystal growth on or off. However, on a negatively charged facet, peptides jostle together on the surface to create clusters that slow or accelerate crystal growth.
“Our results show the effects of peptides on a growing crystal are far more complicated than with simpler, small molecules. The shapes of peptides in solution tend to fluctuate, and depending on the conditions, the complex processes through which peptides stick to surfaces allows them to control crystal growth like a set of ‘switches, throttles and brakes’,” Friddle says. “They can either slow or accelerate growth, or even switch it sharply from on to off with small changes in solution conditions.”
The team plans to use their new approach to investigate fundamental physics of crystal surfaces in solutions and deepen their understanding of how biomolecules and crystals interact. “We believe these results will lay the foundation for better control over technological crystals, biomimetic approaches to materials synthesis, and potential therapies for hard-tissue pathologies,” DeYoreo adds.
More information: The paper “Subnanometer atomic force microscopy of peptide-mineral interactions links clustering and competition to acceleration and catastrophe,” by Raymond Friddle, Matt Weaver, Roger Qiu, Andrzej Wierzbicki, William H. Casey and James J. DeYoreo, appears in Proceedings of the National Academy of Sciences.
Provided by Lawrence Berkeley National Laboratory




















