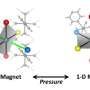
Structure of hydrogen storage molecule solved, once orientation of nearby ions elucidated
(�鶹��ԺOrg.com) -- For nearly a century, nobody knew how the little molecule that’s in the middle of many of today’s hydrogen storage and release concepts was organized. Thanks to an interdisciplinary team of scientists at Pacific Northwest National Laboratory and Los Alamos National Laboratory, the structure of this molecule, known as DADB, has been determined. And DADB’s structure was exactly opposite of what was expected in more ways than one.
"The irony," said Dr. Tom Autrey, the PNNL scientist who led the research, "is that the structure could not be that complex." The challenge was in understanding how one structure, containing a pair of nitrogen and boron atoms surrounded by only 12 hydrogen atoms, stretched and twisted in the solid molecular crystal.
Running cars on fossil fuels presents growing problems, economically, politically, and environmentally. Replacing fossil fuels with hydrogen and fuel cells is an attractive option. Determining the structure of DADB, created at the initial stages when hydrogen is released from the popular hydrogen storage material ammonia borane, allows scientists to accurately model and predict complex, molecular reactions in the solid state. Understanding the subtleties of the structure of DADB also provides insights into developing new materials with the perfect properties to store energy in chemical bonds for efficient fuel cell operations.
The team began by synthesizing the DADB using a new method they developed that allowed the molecular crystal to slowly form at room temperature. They used solid-state nuclear magnetic resonance (NMR) spectroscopy to study the molecule. The NMR spectrum of the molecular crystal was surprisingly different than the NMR spectrum of the molecular complex in solution. The team felt that the hydrogen atoms in the molecular crystal might be influencing the arrangement of atoms.
“Theoreticians couldn’t accurately predict the structure, and experimentalists weren’t getting all the information needed with NMR,” said Dr. Gregory Schenter, a chemical theorist on the study. “So, we used neutron diffraction to see the missing pieces. It took a while, but we got that ‘ah-ha’ moment.”
With the added diffraction data, they could arrange the atoms in a pattern that explained the results they’d seen. “Mark Bowden solved the 100-year-old puzzle,” said Autrey of his PNNL colleague. “He showed how the molecule’s structure was affected by the interactions with the neighboring molecules.”
This research resulted in two different arrangements of borohydride ions (BH4-) giving the molecule its unique twisted structure.
This work is part of a series of broader efforts at PNNL to answer the fundamental questions around how to activate hydrogen for use in catalytic reactions as well as energy storage in chemical bonds for use in fuel cell applications. These fundamental studies are needed if the United States is to develop novel methods to store energy from solar and other intermittent clean energy sources.
More information: Bowden M, DJ Heldebrant, A Karkamkar, T Proffen, GK Schenter, and T Autrey. 2010. “The diammoniate of diborane: Crystal structure and hydrogen release.” Chemical Communications 46, 8564-8566.
Provided by Pacific Northwest National Laboratory