Copper alloy catalysts' surface changes mapped during COâ‚‚ conversion reactions

Gaby Clark
scientific editor

Robert Egan
associate editor

Seoul National University College of Engineering announced that a joint research team has become the first in the world to elucidate the reconstruction mechanism of copper alloy catalysts during electrochemical COâ‚‚ conversion reactions.
The research sheds light on atomic rearrangements in catalyst surface structures during reaction and presents a methodology for predicting and designing actual active sites in operando conditions. The were published in Nature Catalysis and selected as the cover article. The team was led by Professor Young-Chang Joo (Department of Materials Science and Engineering) and Professor Jungwon Park (School of Chemical and Biological Engineering) has, in collaboration with Professors Dae-Hyun Nam (Department of Materials Science and Engineering) and Seoin Baek (KU-KIST Graduate School) at Korea University.
Electrochemical reduction of COâ‚‚ has emerged as a pivotal technology in achieving carbon neutrality, enabling the transformation of greenhouse gas COâ‚‚ into clean and valuable chemical feedstocks. Copper (Cu)-based catalysts are particularly notable for producing high-value multi-carbon compounds such as ethylene (Câ‚‚Hâ‚„) and ethanol (Câ‚‚Hâ‚…OH).
However, single-metal Cu catalysts face intrinsic limitations in selectively controlling the reaction pathways. Alloying Cu with other metals to create multiple active sites has been a strategy to enhance product selectivity and catalytic efficiency. While previous studies have focused on synthetic control of surface composition and nanostructure, they have overlooked dynamic changes under actual reaction conditions.
During CO₂ electroreduction, dynamic reconstruction of the catalyst surface—due to repeated metal dissolution and redeposition—becomes inevitable. This often disrupts the finely tuned surface structure originally designed for optimal activity, making it difficult to predict or optimize catalyst performance.
The complexity is amplified in bimetallic or multimetallic systems, where the roles of different metal species in the reconstruction process remain largely unexplored. Therefore, understanding and controlling reconstruction phenomena in such systems is a critical step toward advancing COâ‚‚ reduction catalyst design.
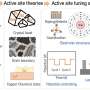
The researchers established a material selection map based on the oxophilicity and miscibility between Cu and the secondary metal X, and fabricated four representative Cu–X alloy catalysts (X = Ag, Fe, Zn, Pd). These catalysts were integrated into gas-diffusion electrodes and subjected to industrially relevant high-current CO₂ electroreduction conditions to induce surface reconstruction.
-

In-situ liquid-phase TEM observation of nanoparticle formation and growth on Cu–Ag thin films. Credit: Nature Catalysis -

Schematic of the reconstruction mechanism governed by intermediate adsorption and metal miscibility. Credit: Nature Catalysis
Using cross-sectional transmission electron microscopy (TEM), they succeeded in capturing the surface structure changes—an achievement that overcomes the limitations of previous low-current-density catalyst reconstruction studies.
Notably, Cu–Ag catalysts exhibited surface formation of Cu nanoparticles during the reaction, while Cu–Zn alloys maintained a uniform elemental distribution. Despite similar CO-producing capabilities, the reconstruction behavior yielded stark differences in product selectivity. In Cu–Ag, the Cu nanoparticles promoted further conversion of CO intermediates to ethanol, preserving high ethanol selectivity even at high Ag content. In contrast, Cu–Zn showed a decline in ethanol production due to a lack of Cu-rich active sites, favoring CO desorption instead.
Furthermore, through in-situ liquid-phase TEM, the researchers directly observed the nucleation and growth of Cu nanoparticles and identified a selective dissolution–redeposition mechanism induced by intermediate adsorption. They also demonstrated that the rearrangement behavior of redeposited atoms was determined by the miscibility of alloy components. Crucially, they applied a pulsed potential strategy to control dissolution–redeposition kinetics and successfully shifted product selectivity in Cu–Zn from CO toward ethanol.
This study provides a "design map" for understanding and predicting surface reconstruction in Cu-based bimetallic catalysts, offering a theoretical foundation for designing catalysts that dynamically adapt during operation. The design principles may be generalized to more complex multimetallic systems, ultimately advancing the commercialization of COâ‚‚ conversion technologies by improving catalytic performance and durability.
Professor Young-Chang Joo remarked, "This is the first study to systematically unveil the dynamic reconstruction behavior of alloy catalysts during electrochemical COâ‚‚ reduction. By moving beyond optimization of synthesis conditions and incorporating in-situ structural evolution into catalyst design, we present a new paradigm in high-performance catalyst development."
Lead author Intae Kim, a combined Master-Ph.D. student in the SNU Department of Materials Science and Engineering, plans to expand the framework of dynamic catalyst design by investigating the kinetics of reconstruction under pulsed COâ‚‚ reduction conditions.
More information: Intae Kim et al, Unveiling the reconstruction of copper bimetallic catalysts during CO2 electroreduction, Nature Catalysis (2025).
Journal information: Nature Catalysis
Provided by Seoul National University