Sticky particles don't succumb to sunlight, an insight that could help refine pollution models

In an unexpected twist, an atmospheric particle's stickiness protects it from sunlight when the air is cool and dry. The particle's sticky nature, or viscosity, slows down the motion of molecules inside. The result is that the molecules that absorbed sunlight's energy cannot easily reach their reaction partners and the energy is dispersed into heat instead. This study is the first to show the link between viscosity and sunlight's inability to degrade the particle, and as such, it was chosen to grace the cover of an upcoming Â鶹ÒùÔºical Chemistry Chemical Â鶹ÒùÔºics.
The research was led by scientists at the University of California, Irvine and involving scientists at the University of British Columbia and Pacific Northwest National Laboratory.
The findings suggest air pollutants trapped inside the particles in polar regions or at high altitudes will have longer photodegradation lifetimes than those in warm, humid regions. By describing reactions inside and on surfaces of atmospheric particles produced by industrial and natural processes, the team's research could lead to more detailed air pollution models.
Atmospheric chemistry is almost entirely driven by sunlight. For decades, scientists have uncovered the secrets of sunlight-driven chemical reactions that happen with gaseous molecules in the air. However, the reactions inside and on surfaces of atmospheric particles remain unexplored. To address this situation, the team explored the effect of environmental conditions on sunlight-driven or photodegradation rates of atmospherically relevant pollutants embedded in secondary organic aerosols.
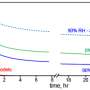
The scientists examined particles made from oxidation products of three different chemicals: alpha-pinene, limonene, and ammonia-exposed limonene. The pollutant of choice was 2,4-dinitrophenol.
They found that the photodegradation rates of the pollutant were slower at temperatures and humidity levels that made the particles more viscous.
Why? Inside the particles, certain molecules absorb light and then react by abstracting hydrogen atoms from nearby molecules. The reaction converts the molecules inside the particles into various products. The viscous nature of the particle changes the reaction rate by slowing down the reagent molecules. The more viscous the particle, the longer it takes to abstract the hydrogen allowing for energy to dissipate into heat and keep the molecules intact.
The findings show toxins trapped inside viscous particles, which are more abundant in cold, dry parts of the atmosphere, may take longer to decompose than expected.
The research was conducted using experimental resources available through the user program at EMSL, a Department of Energy Office of Science national scientific user facility.
The team is continuing their collaborative efforts to reveal how temperature, humidity, and other conditions influence the photodegradation of compounds that affect the atmosphere.
More information: Mallory L. Hinks et al. Effect of viscosity on photodegradation rates in complex secondary organic aerosol materials, Â鶹ÒùÔº. Chem. Chem. Â鶹ÒùÔº. (2016).
Journal information: Â鶹ÒùÔºical Chemistry Chemical Â鶹ÒùÔºics
Provided by Pacific Northwest National Laboratory