September 15, 2017 report
Metal-catalyzed addition of saturated carbon into C-C bonds: A relevant reaction for the synthesis type II polyketides
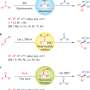
![Ruthenium-catalyzed cyclobutenone-diol [4+2]cycloaddition via C-C bond activation: A gateway to type II polyketide natural products. Credit: (c) Science (2017). DOI: 10.1126/science.aao0453 Metal-catalyzed addition of saturated carbon into C-C bonds: A relevant reaction for the synthesis type II polyketides](https://scx1.b-cdn.net/csz/news/800a/2017/59bb97a4ea5cd.jpg)
(Â鶹ÒùÔº)—Type II polyketides are a class of compounds found in nature as secondary metabolites derived from microorganisms. Their complex structures are characterized by fused polycyclic aromatic rings decorated with a profusion of ketones and hydroxyl groups. Many common antibiotics, anti-fungal, and anti-cancer drugs are type II polyketides or are derivatives of these naturally-occurring compounds. However the "laboratory synthesis" of type II polyketides is often challenging; as in living systems, it is a highly selective multi-step enzymatic process.
Researchers from the University of Texas led by Professor Michael J. Krische have developed a ruthenium-catalyzed cycloaddition of benzocyclobutenones and diols that directly deliver substructures found in diverse type II polyketides. Their catalytic reactions involve successive insertion of saturated diol carbon-hydrogen (C-H) bonds into a carbon-carbon (C-C) bond by way of a ruthenacycle intermediate. Their report appears in Science.
This research builds on prior studies by this group on metal-catalyzed C-C bond forming reactions of alcohols via hydrogen transfer—a modern twist on carbonyl addition chemistry. Whereas classical carbonyl additions typically involve the reaction of pre-formed organometallic reagents with an aldehyde or ketone (e.g., a Grignard reaction), in the current paper these reactive species are generated transiently and in duplicate. The ruthenium catalyst inserts into a stained C-C bond to form a ruthenacycle bearing TWO carbon-ruthenium bonds, and the catalyst converts the diol to a transient diketone. These species engage in double-carbonyl addition that results in cycloaddition. The net result is the formal insertion of two saturated diol C-H bonds into a saturated C-C bond.
As a model reaction to test their concept, the authors reacted benzocyclobutenone with trans-cyclohexane 1,2-diol in the presence of their ruthenium catalyst. After tweaking the reaction conditions, they obtained an 88% yield of the tricyclic product bearing a bridgehead diol, which formed exclusively as the syn-stereoisomer.
To examine the versatility of their conditions, they reacted a range of benzocyclobutenones with a variety of 1,2-diols. Several of the diols tested had fused rings. This resulted in the formation of cycloadducts with tetracyclic substrates found in several type-II polyketides, including angucycline natural products with congested "bay-region" architectures. Notably, each of the reactions displayed complete regio- and stereoselectivity. Additionally, the reaction tolerated halide substitution on the benzene ring allowing for further functionalization.
Studies to elucidate the reaction mechanism showed that the cycloaddition can be performed in a redox-independent manner. That is, the benzocyclobutenone can react with a diol, a ketol, or a dione producing the same product as a single regio- and stereoisomer. While the reaction of the diol is oxidative and requires a slight excess of benzocyclobutenone, the reaction of the ketol is redox-neutral and can be performed with equal amounts of benzocyclobutenone and ketol. The reductive reaction of the dione requires the addition of 2-propanol to act as a reductant
Beyond broadening access to type II polyketides, the reactivity embodied by these studies has broader implications in the field of chemical synthesis.
Dr. Michael Krische told Â鶹ÒùÔºOrg, "In contrast to progress recently made in the field of C-H activation, the development of catalysts that modify C-C sigma-bonds has lagged behind, and is largely restricted to intramolecular reactions that insert tethered Ï€-bonds, often requiring directing-groups. Hydrogen-transfer offers a broad, new approach to the functionalization of C-C bonds using abundant, renewable alcohols as coupling partners."
More information: Matthias Bender et al. Ruthenium-catalyzed insertion of adjacent diol carbon atoms into C-C bonds: Entry to type II polyketides, Science (2017).
Abstract
Current catalytic processes involving carbon-carbon bond activation rely on π-unsaturated coupling partners. Exploiting the concept of transfer hydrogenative coupling, we report a ruthenium(0)-catalyzed cycloaddition of benzocyclobutenones that functionalizes two adjacent saturated diol carbon-hydrogen bonds. These regio- and diastereoselective processes enable convergent construction of type II polyketide substructures.
Journal information: Science
© 2017 Â鶹ÒùÔº