Lifespan of fuel cells maximized using small amount of metals
Fuel cells are a key future energy technology emerging as eco-friendly and renewable energy sources. In particular, solid oxide fuel cells composed of ceramic materials can directly convert fuels such as biomass, LNG, and LPG to electric energy. KAIST researchers have described a new technique to improve the chemical stability of electrode materials that can extend the lifespan by employing minimal amounts of metals.
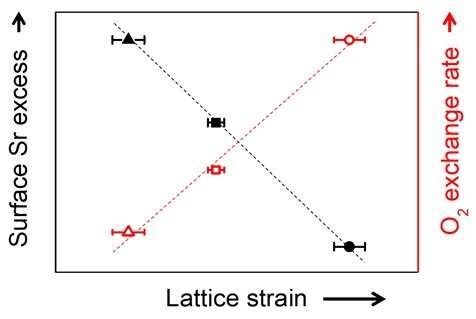
The core factor that determines the performance of solid oxide fuel cells is the cathode at which the reduction reaction of oxygen occurs. Conventionally, perovskite structure oxides (ABO3) are used in cathodes. However, despite the high performance of perovskite oxides at initial operation, performance degrades with time, limiting their long-term use. In particular, the condition of a high-temperature oxidation state required for cathode operation leads to a surface segregation phenomenon in which second phases such as strontium oxide (SrOx) accumulate on the surface of oxides, resulting in a decrease in electrode performance. The detailed mechanism of this phenomenon and a way to effectively inhibit it has not been suggested.
Using computational chemistry and experimental data, Professor WooChul Jung's team at the Department of Materials Science and Engineering observed that local compressive states around the Sr atoms in a perovskite electrode lattice weakened the Sr-O bond strength, which in turn promote strontium segregation. The team identified local changes in strain distribution in perovskite oxide as the main cause of segregation on the strontium surface. Based on these findings, the team doped different sizes of metals in oxides to control the extent of lattice strain in cathode material and effectively inhibited strontium segregation.
Professor Jung said, "This technology can be implemented by adding a small amount of metal atoms during material synthesis, without any additional process." He continued, "I hope this technology will be useful in developing high-durable perovskite oxide electrode in the future."
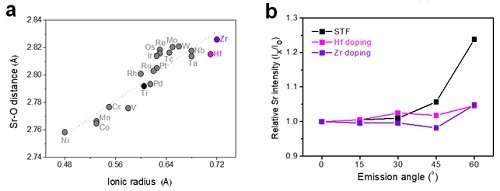
More information: Bonjae Koo et al, Enhanced oxygen exchange of perovskite oxide surfaces through strain-driven chemical stabilization, Energy & Environmental Science (2017).
Journal information: Energy & Environmental Science




















