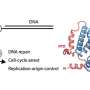
Radiotherapy: Repairing proteins in search of the ring to protect DNA
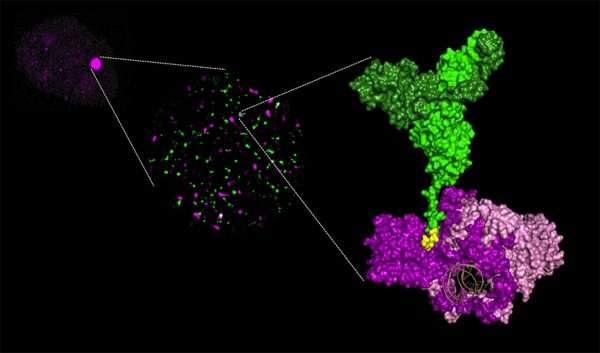
To understand why some cancer cells resist radiotherapy, an international team of researchers has used crystallography to "photograph" the first moments of the molecular ballet that allows these cells to repair their DNA. The study involved teams from the CEA, CNRS, PROXIMA-1 beamline at SOLEIL, University of Paris-Sud, Gustave Roussy, Aix-Marseille University and University Paul Sabatier—Toulouse III. It was published in Nature Structural & Molecular Biology.
Radiotherapy is a critical tool in cancer treatment. Prescribed in one in two cases (i.e. 200,000 cases per year in France), the rays used in radiotherapy destroy cancer cells by fragmenting their DNA. However, in tumours, some cells can resist treatment by repairing the breaks in their DNA. To increase the effectiveness of radiotherapy on the tumour, for instance by inhibiting DNA repair in the tumour, researchers first need to have a detailed understanding of how these repair mechanisms work.
In irradiated cells, a protein complex assembles around a ring-shaped protein known as Ku, which quickly encircles the ends of DNA breaks. Through this ballet, the ends of breaks are repaired by joining together.
Researchers studied the first phase of this dance, with Ku at the centre, and particularly the involvement of APLF and XLF, two proteins that work with Ku. Using X-ray crystallography to view the protein complexes at an atomic scale, they produced snapshots of interactions between Ku/APLF and between Ku/XLF. For the first time ever, these images show that each of the two partners comes into contact with Ku on distinct sites. Researchers showed that if these sites are changed, breaks are no longer properly repaired and the cells much less survive after they are irradiated.
In the long term, precise knowledge of the contact points between the DNA break repair catalysts could lead to the development of molecules that would perfectly adjust to these sites. By preventing the assembly of the repair mechanism in tumours, these molecules could potentiate radiotherapy treatments.
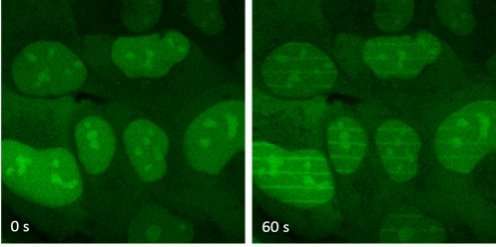
More information: Clement Nemoz et al. XLF and APLF bind Ku80 at two remote sites to ensure DNA repair by non-homologous end joining, Nature Structural & Molecular Biology (2018).
Journal information: Nature Structural & Molecular Biology
Provided by CNRS