July 30, 2019 feature
Bone tissue engineering—nano-glue polymer membranes for robust bone regeneration

Thamarasee Jeewandara
contributing writer
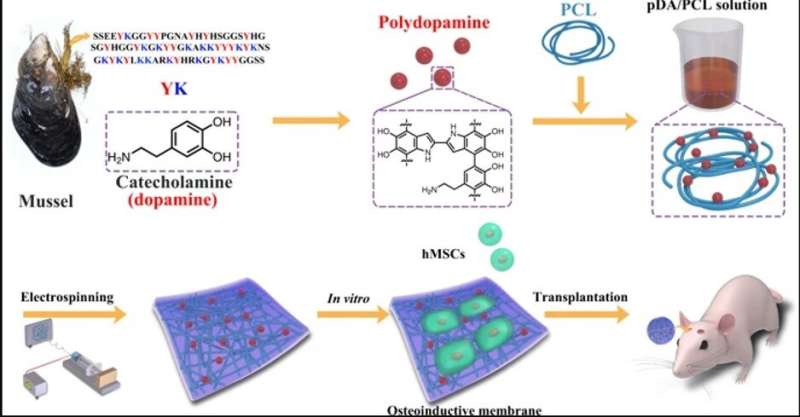
In a new study now published on NPG Asia Materials, bioengineers report the development of a new fibrous membrane with to repair bone defects in the skulls of mice. For this, they incorporated mussel-inspired protein as a promising compound to tether biological substances to the membrane surfaces, much like adhesive proteins in mussels. In the work, Yi Deng and a research team in the interdisciplinary departments of chemical engineering, mechanical engineering, materials technology, center for future materials and regenerative medicine in China and Australia, coated the biocompatible membranes with polydopamine nanoparticles to form many topological sites for calcium attachment and bone defect repair.
The team incubated the uncoated and coated membranes with stem cells isolated from bone marrow and implanted the membranes to regenerate skull bone defects in live mice. After a 2-month translational study, they revealed the ability of the sticky membranes to direct stem cells to produce significantly higher quantities of bone, compared with uncoated membranes. Bone defects and injuries can commonly occur at the microscopic level as congenital defects, due to accidents or as age-related degenerative disease. Most bone defects cannot be repaired spontaneously by self-healing mechanisms leading to an urgent requirement to develop robust biomaterials that facilitate bone repair in and bone tissue engineering.
Bioengineers can manipulate stem cell differentiation to form mature via (GTR) on surface membranes for optimized, large-scale bone regeneration. In materials science and advanced functional materials, have received massive attention for such guided tissue engineering strategies due to several biocompatible advantages, including:
- Biomimicry for
- Large surface area to facilitate cell adhesion and growth
- The ability to and accelerate the osteogenic potential of numerous stem cell lines (from mouse, rat and human species).
Materials scientists classify synthetic GTR materials into two main categories as (1) bioabsorbable and (2) nonabsorbable materials; where nonabsorbable materials must be removed after implantation via a second surgery, causing increased healthcare expenses, while compromising newly generated tissue. In contrast, biodegradable membranes such as , (PLGA) or PCL are preferred for clinical implantations, although biological complications have seriously impeded .
In the present work, therefore, Deng et al. used the bioinspired adhesive protein as a 'material-independent' and facile surface coating, engineering strategy based on . can facilitate osteoblastic differentiation of stem cells on a variety of substrates as a nanoscale biomaterials coating, to and of human . Nevertheless, PDA nanolayers can easily delaminate from surfaces to induce or as adverse effects. Deng et al. implemented specific experimental steps to overcome the existing limitations and develop a new biocompatible and biodegradable membrane in the lab. The newly engineered biomaterial or fibrous membrane will provide a favorable niche to steer the fates of local stem cells to form osteoblasts for bone regeneration.
Step one: Engineering PDA-included PCL (PDA/PCL) with electrospinning
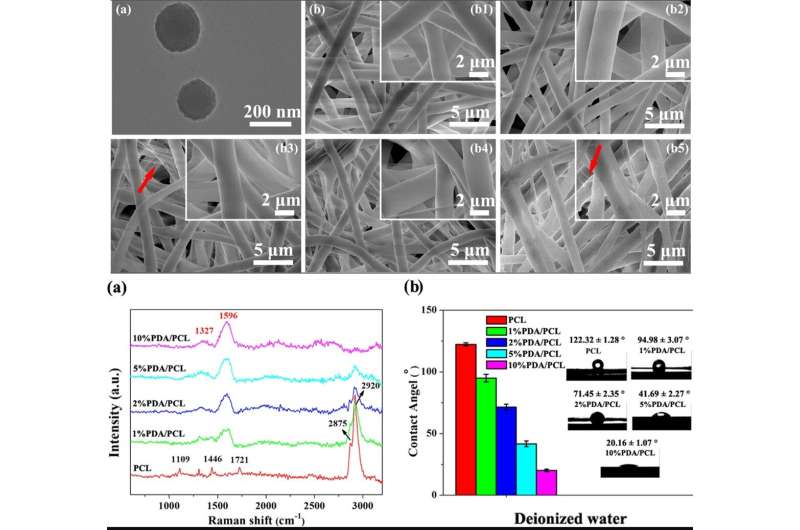
The scientists used catechol chemistry for PDA/PCL fibrous membrane fabrication and synthesis. They uniformly dispersed the PDA nanoparticles (NPs) in PCL via continuous sonication and vortexing to form the fibrous membranes via electrospinning. Deng et al. used a and observed surface properties of the random, micron-sized fibrous network. Comparatively, the pure PCL electrospun membranes remained smooth, whereas the integration of PDA NPs made the fiber surfaces rough. The scientists confirmed the new surface chemistry using and . The biomaterials were hydrophilic allowing protein adsorption and cell attachment. The researchers conducted to verify surface wettability and improved hydrophilicity after PDA modification compared to the pure, unmodified PCL membranes.
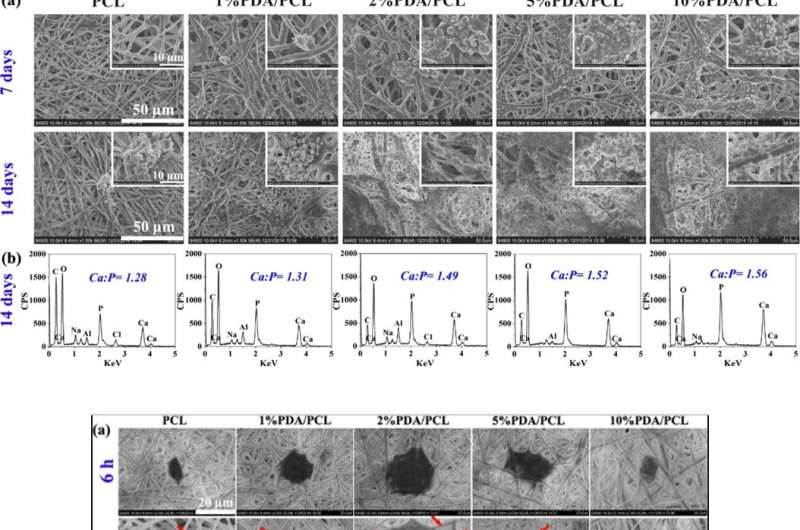
Step two: Surface characterization studies
Since bioactive membranes can integrate with surrounding (bone tissue), Deng et al. assessed the formation of bone apatite layers on the surface of biomaterials immersed in (SBF) solution. After 7 days of immersion, the scientists observed clustered nodular aggregates on the PDA/PCL membranes, which increased dramatically by day 14. Comparatively, the control sample of pristine unmodified PCL retained Ca-P deposits at seven days, with islands of apatite by day 14. As the PDA content increased, therefore, the amount of apatite deposited on the surface increased. Deng et al. used the material characterization data to validate enhanced in vitro bioactivity of PDA/PCL membranes compared to the pure PCL control.
Step three: Biofunctionalization studies
The scientists assessed the cytocompatibility (cell biocompatibility) of the engineered PDA/PCL membranes relative to cell adhesion, spreading, and human (hMSCs) proliferation. The hMSCs largely exist in bone marrow to assist tissue repair during injury. After 6 hours of cell culture, the hMSCs with round cellular shapes did not adhere well on pure PCL but expressed filopodia for membrane surface attachment on three variants of PDA/PCL membranes. Using and , Deng et al. showed that the content of PDA NPs significantly affected the number of viable cells attached on the surface, and observed optimal surface properties with the 2 percent PDA/PCL group in the work.
The research team determined the optimal formula to engineer PDA/PCL membranes for guided bone tissue regeneration by determining the activity and calcium matrix production of hMSCs with (ARS) staining. Cell growth and was low when the amounts of PDA NPs were either high or low, because low concentrations did not trigger cell growth, whereas high concentrations were toxic in the study.
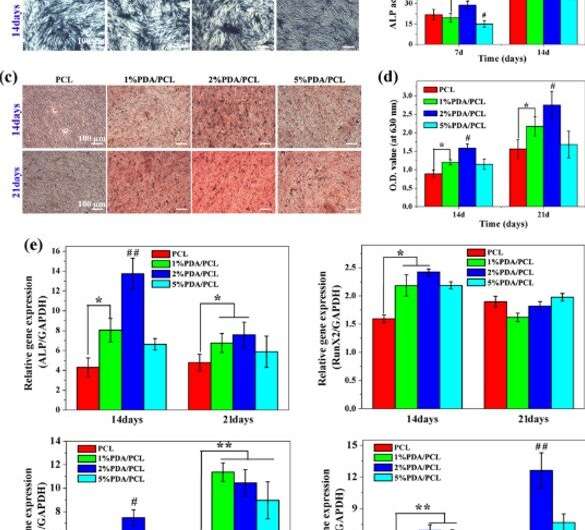
Since investigations at the molecular level are a powerful tool in biomaterials for bioengineering, Deng et al. investigated the interplay between hMSCs and engineered membranes using molecular tools. For this, they monitored the expression of osteogenesis-related genes , , and in hMSCs cultured on the membranes. At 7 days they observed substantial levels of ALP gene expression on the 2 percent PDA/PCL sample.
By day 14, the level of Runx2 gene expressed on 1 and 2 percent PDA/PCL groups significantly outmatched the pure PCL group. However, by 21 days, the scientists did not observe a discernable difference among the four groups. They verified the observations using staining and chose the 2 percent PDA/PCL membranes for optimal induction of hMSCs to differentiate into mature osteoblasts.
Step four: Translational study
Guided by the data from in vitro experiments, Deng et al. investigated the in vivo biofunctionality of the microfibrous membrane using an animal model. For this, they created critical-sized bone defects on mice skulls and placed fibrous membranes to cover the defects, followed by bone formation tests using , histological analyses and fluorescent labeling; four to eight weeks after implantation.
When they examined the 3-D images of the micro-CT skulls, the 2 percent PDA/PCL membrane offered the largest areas of new bone formation, with considerable expansion to the center of the bone defect. The scientists obtained a higher content of calcified matrix and bone remodeling in the 2 percent PDA/PCL membranes for phenomenal osteoconductive integration.
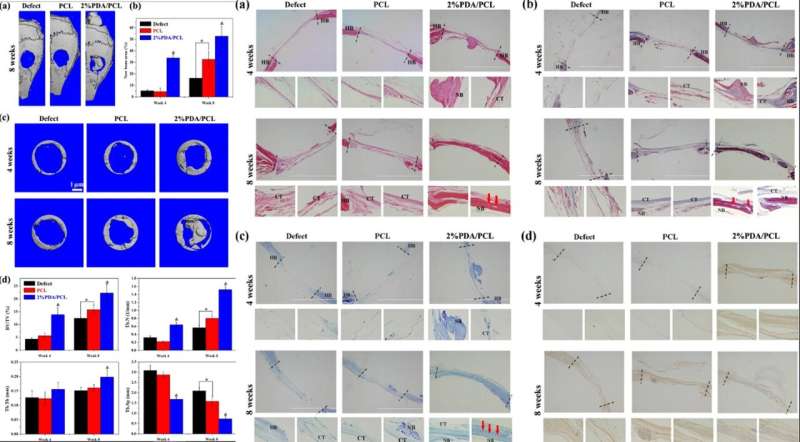
The revealed fibrous tissue in the cavities of the PCL group, with comparatively visible bone restructuring in the 2 percent PDA/PCL group. The scientists also observed bone with abundant vascularization after 8 weeks post-operation in the 2 percent PDA/PCL groups. They conducted further staining with Masson, toluidine blue and immunohistochemistry (IHC) staining to identify new bone and collagen formation in depth. The combined histological data revealed that employment of PDA NPs in engineered fibrous membranes significantly boost bone regeneration, supporting the hypothesis that in vitro osteodifferentiation was also effective in vivo.
In this way, Yi Deng and co-workers bioengineered co-electrospun PDA NPs with a bioinert synthetic polymer to construct bioinspired, flexible and osteopromotive PDA/PCL fibrous membranes for bone tissue engineering applications in regenerative medicine. The amount of PDA NPs included in the composite significantly improved the chemical composition, fiber size and mechanical properties of the developed membranes. Both in vitro experiments and in vivo data validated the ability for new bone formation with 2 percent PDA/PCL constructs compared with pure PCL. The engineered PCL/PDA membranes are osteoconductive and easy to transplant with great potential for GTR applications.
Written for you by our author —this article is the result of careful human work. We rely on readers like you to keep independent science journalism alive. If this reporting matters to you, please consider a (especially monthly). You'll get an ad-free account as a thank-you.
More information: Yi Deng et al. Bioinspired and osteopromotive polydopamine nanoparticle-incorporated fibrous membranes for robust bone regeneration, NPG Asia Materials (2019).
Rabih O. Darouiche. Treatment of Infections Associated with Surgical Implants, New England Journal of Medicine (2004).
Rajeswari Ravichandran et al. Precipitation of nanohydroxyapatite on PLLA/PBLG/Collagen nanofibrous structures for the differentiation of adipose derived stem cells to osteogenic lineage, Biomaterials (2011).
H. Lee et al. Mussel-Inspired Surface Chemistry for Multifunctional Coatings, Science (2007).
Journal information: New England Journal of Medicine , Biomaterials , Science
© 2019 Science X Network