You're not so tough, h-BN
Hexagonal-boron nitride is tough, but Rice University scientists are making it easier to get along with.
Two-dimensional h-BN, an insulating material also known as "white graphene," is four times stiffer than steel and an excellent conductor of heat, a benefit for composites that rely on it to enhance their properties.
Those qualities also make h-BN hard to modify. Its tight hexagonal lattice of alternating boron and nitrogen atoms is highly resistant to change, unlike graphene and other 2-D materials that can be easily modified—aka functionalized—with other elements.
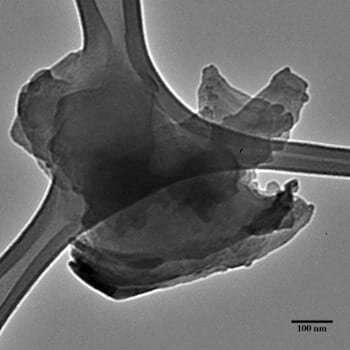
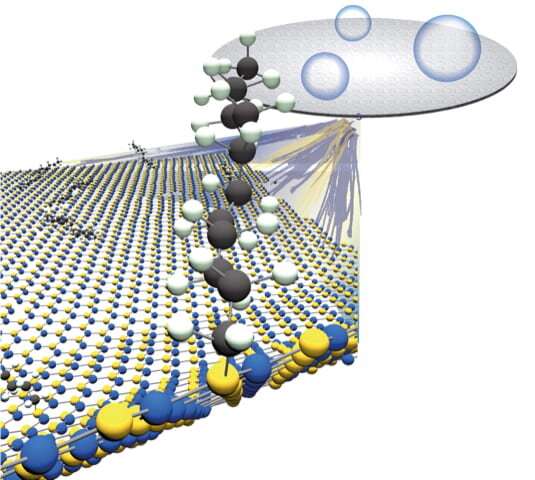
The Rice lab of chemist Angel Martà has published a protocol to enhance h-BN with carbon chains. These turn the 2-D tough guy into a material that retains its strength but is more amenable to bonding with polymers or other materials in composites.
The lab's paper in the American Chemical Society's Journal of Â鶹ÒùÔºical Chemistry suggests h-BN can be made more dispersible in organic solvents as well. Martà and his team modified the Billups-Birch reaction process they had successfully used to alter boron nitride nanotubes to attack the defenses of h-BN and covalently attach carbons.
Birch reduction, discovered in the 1940s and enhanced in 2004 by Rice Professor Emeritus of Chemistry Edward Billups to functionalize carbon nanotubes, frees electrons to bind with other atoms. In the Rice process, Martà and his team can control the amount of h-BN functionalization by varying the amount of lithium in the reaction.
Lithium is an alkali metal that sheds free electrons when combined with liquefied ammonia. Mixed with h-BN flakes and a carbon source, 1-Bromododecane in this case, the reaction produces an alkyl radical, a chemical species that reacts with h-BN and makes a bond.
Martà said it's the best method found so far to modify h-BN, which resists change even under high temperatures. "You take a little bit of graphite and put it in a furnace at 800 degrees (Celsius), and it will be gone," he said. "You take hexagonal-boron nitride and do the same, and it will still be there smiling at you.
"That gives you an idea of how stable it is, and that's the problem we wanted to address," Martà said. "The material is good for certain applications, but to control its properties for manufacturing, you have to graft different groups onto the surface."
He said a 20-to-1 molar ratio of lithium to h-BN optimized the process of grafting carbon chains to the surface and edges.
Because the base h-BN remains stable under high temperatures, it can be returned to its pristine state by simply burning off the functional chains.
While h-BN is naturally hydrophilic (water-attracting), the functional carbons make them nearly superhydrophobic (water-avoiding), a good property for making protective films, Martà said. But even when enhanced, the flakes remain amenable to dispersion in non-polar solvents.
Martà said his group is exploring what other kinds of molecules can be grafted onto white graphene. "What about benzene groups? What about ethers? What about groups that will make it compatible with other materials?
"There's a lot of interest in making composite materials between h-BN, boron nitride nanotubes and polymers," he said. "Ultimately, we'd like to graft different groups onto h-BN and build a library, kind of a toolbox, of functional groups that can be used with these materials."
More information: Carlos A. de los Reyes et al, Tunable Alkylation of White Graphene (Hexagonal Boron Nitride) Using Reductive Conditions, The Journal of Â鶹ÒùÔºical Chemistry C (2019).
Journal information: Journal of Â鶹ÒùÔºical Chemistry A , Journal of Â鶹ÒùÔºical Chemistry C
Provided by Rice University