December 30, 2019 feature
New class of crosslinker-free nanofiber biomaterials from Hydra nematocyst proteins

Thamarasee Jeewandara
contributing writer
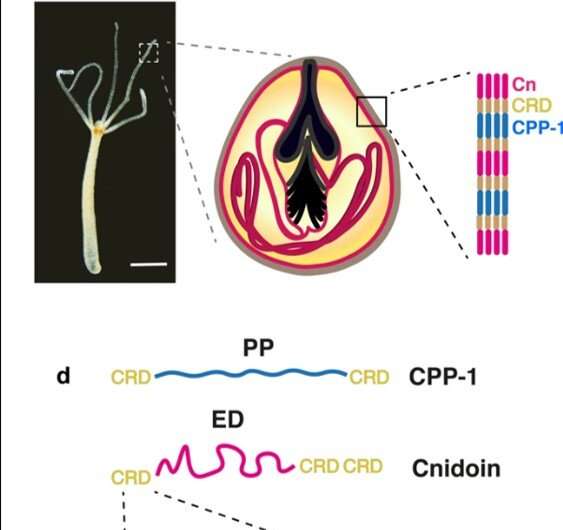
Nematocysts are stinging organelles of that have remarkable mechanical properties to undergo 50 percent volume changes during explosive exocytosis (process by which cells excrete waste and large molecules), while withstanding osmotic pressures beyond 100 bar. Researchers had recently identified two novel protein components that built up the nematocyst wall in Hydra to include (1) a cnidarian proline-rich protein-1 (CPP-1) with a rigid polyproline motif, and (2) an elastic Cnidoin possessing a silk-like domain. In a new study, now on Scientific Reports, Theresa Bentele and a team of researchers in the departments of Medicine, Molecular Evolution and Genomics and the Institute of �鶹��Ժical Chemistry in Germany, Australia and Japan, expressed recombinant Cnidoin and CPP-1 proteins in Escherichia coli.
They compared the of spontaneously crosslinked bulk proteins with that of isolated . The researchers systematically optimized the fabrication of uniform protein nanofibers using and preparative conditions. Both fibers remained stable even after rigorous washing and immersion in bulk water, due to simultaneous crosslinking of cysteine-rich domains. The resulting nanofibers were clearly different from other protein nanofibers that were unstable without chemical crosslinker procedures. After quantitative assessment of mechanical properties, they examined applications of Cnidoin and CPP-1 nanofibers to promote the growth of .
Hydra comprise four variants that develop in the body column of polyps in specialized cells known as . The outstanding mechanical toughness of the capsule wall structure make nematocysts unique to form bioinspired materials in the lab. The capsule contains protein complexes crosslinked by intermolecular between (CRD), which can be used as a versatile crosslinker to create linear or branched polymers among diverse proteins. The scientists had already identified two new capsule proteins including and while studying Hydra nematocysts in their previous work. The potential to combine elastic Cnidoin and rigid CPP-1 proteins was a promising strategy to design new biomaterials capable of forming stable structures with spontaneous crosslinking to realize outstanding flexibility and toughness, similar to biological . Synthetic bioinspired protein nanofibers have gained increasing attention as an artificial matrix to culture stem cells for tissue engineering applications. Electrospinning offers a common method to fabricate such fibers using , and . The thin fiber products have multiple applications in .
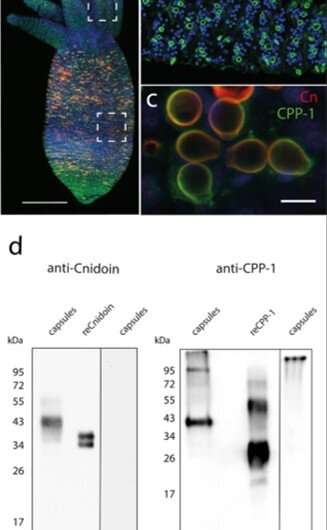
In the present work, Bentele et al. introduced a new class of synthetic crosslinker-free nanofibers based on the Hydra nematocyst proteins CPP-1 and Cnidoin using . Based on the spontaneous crosslinking capacity of CRDs they systematically optimized the preparative conditions to bioengineer crosslinker-free protein nanofibers that are stable under water with potential applications for human stem cell culture. The research team obtained representative immunofluorescence images of a Hydra with CPP-1 (green) and Cnidoin (red) antibodies to co-localize the proteins in the capsule wall. The images indicated the presence of Cnidoin as more densely packed within mature nematocyst walls compared to CPP-1. Thereafter, Bentele et al. used methods to identify the isolated native nematocyst capsules and (proteins expressed in other organisms); which they produced in E. coli. The results indicated considerable of CPP-1 in Hydra. They confirmed the results using the CPP-1 protein as expressed in E. coli and deduced both CPP-1 and Cnidoin to be structural proteins of the nematocyst wall integrated during formation or morphogenesis.
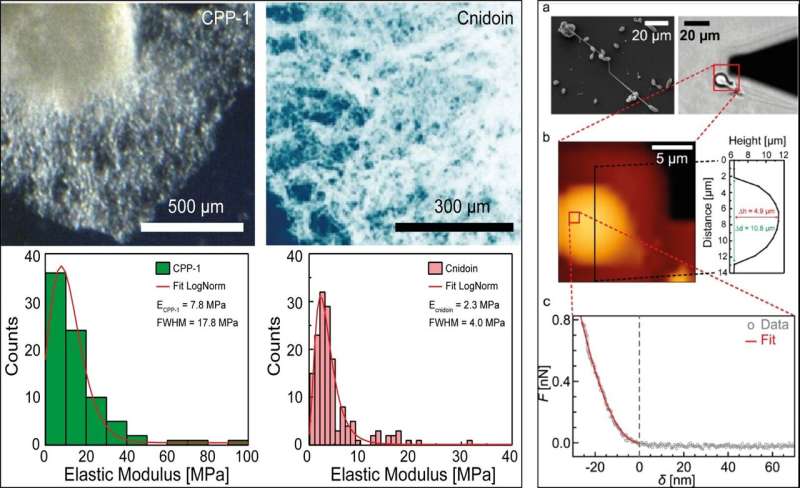
The researchers then tested the mechanical properties of Hydra nematocysts and bulk proteins using (SEM) and (AFM). The scientists extracted the distribution of the and further measured the elasticity of purified recombinant CPP-1 (reCPP-1) and Cnidoin (reCnidoin) expressed in E. coli. They then optimized nanofiber production by introducing 900 kDa to the pure solution to of the product. The team investigated the influence of relative humidity—which significantly affected the quality of nanofibers, while the ionic strength or conductivity of spinning solutions showed no influence on the nanofibers.
Based on the preliminary materials development and characterization results, Bentele et al. fabricated protein nanofibers by electrospinning the protein-PEG solution on glass coverslips. The freshly spun recCPP-1-PEG nanofibers showed a uniform width and height across a 50 x 50 µm2 area and displayed a uniform elastic modulus. The team then measured the , obtained an and a characteristic for reCPP-1 and reCnidoin nanofibers (a) in air, (b) in air after washing with water, and in (c) physiological buffer solution. They could remove PEG by washing with water to obtain a significantly decreased fiber thickness for reCnidoin nanofibers, although the dimensions were less pronounced compared to reCPP-1 after water treatment.
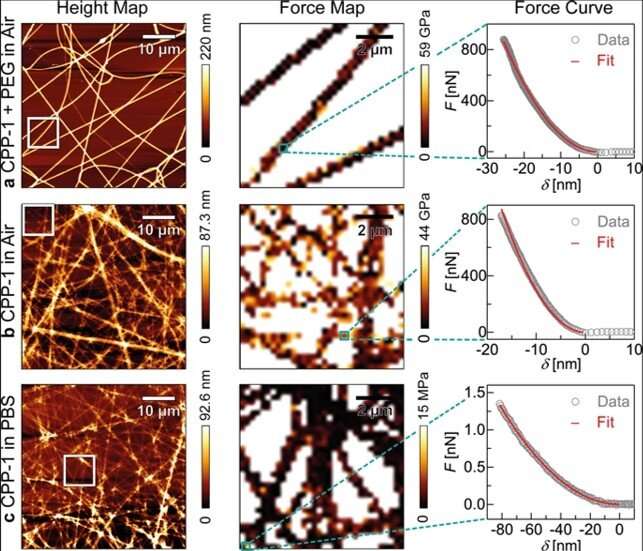
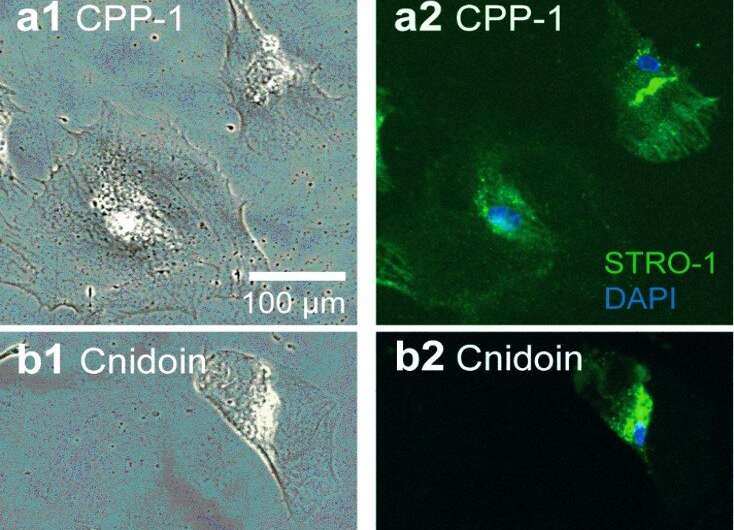
However, the fibers did not dissolve entirely after washing with water and retained their elastic moduli. The results suggest that the two recombinant proteins can establish stable nanofibers by spontaneously forming disulfide bonds between CRD (cysteine-rich-domain) termini. The recombinant Hydra nematocyst proteins produced in this work also formed uniform and stable nanofibers through naturally occurring CRDs within air and in physiological buffer. The team examined the applications of these nanofibers with stable human mesenchymal stem cell culture for 20 days of incubation, during which approximately 95 percent of cells showed cell growth and viability on the new bioinspired materials.
In this way, Theresa Bentele and colleagues proposed a new synthetic crosslinker-free nanofiber biomaterial, bioinspired by the nematocyst capsule proteins of Hydra. They expressed recombinant proteins of two recently identified CPP-1 and Cnidoin nematocyst capsule proteins within E. coli and prepared nanofibers via electrospinning. As a result of the cysteine-rich domains (CRD), the electrospun fibers could spontaneously crosslink via disulfide bonds. The reCPP-1 and reCnidoin recombinant proteins formed uniform nanofibers that were stable in water directly after electrospinning. The new material constructs demonstrated potential as biocompatible materials inspired by the tough and elastic Hydra nematocyst structure.
Written for you by our author —this article is the result of careful human work. We rely on readers like you to keep independent science journalism alive. If this reporting matters to you, please consider a (especially monthly). You'll get an ad-free account as a thank-you.
More information: Theresa Bentele et al. New Class of Crosslinker-Free Nanofiber Biomaterials from Hydra Nematocyst Proteins, Scientific Reports (2019).
A. Lazaris. Spider Silk Fibers Spun from Soluble Recombinant Silk Produced in Mammalian Cells, Science (2002).
Mengyan Li et al. Electrospun protein fibers as matrices for tissue engineering, Biomaterials (2005).
Journal information: Scientific Reports , Science , Biomaterials
© 2019 Science X Network




















