February 20, 2020 feature
Microchannel network hydrogel-induced ischemic blood perfusion connection

Thamarasee Jeewandara
contributing writer
Controlled angiogenesis (growth of new blood vessels) at damaged sites is an unresolved issue in the clinical setting. Most attempts include local treatment with pro-angiogenic molecules,Jo although the approach can induce inflammatory coupling, tumorous vasculature activation and off-target circulation. Bioengineers predict that a three-dimensional (3-D) structure could guide desirable biological functions at the implant site, without any therapeutic treatment. In a new report on Nature Communications, Jung Bok Lee and a research team at the Departments of Medical Engineering and Biomedical Engineering in the U.S. and the Republic of Korea generated such microchannel networks in a gelatin hydrogel.
The product overcame the diffusion limit of nutrients and oxygen in a 3-D setup. They implanted the construct in mouse and porcine (pig) (inadequate blood supply to the hindlimb) to rescue severely damaged tissues via the ingrowth of neighbouring host vessels with microchannel perfusion. They guided the process using microchannel-sized specific regenerative macrophage polarization for functional recovery of endothelial cells. Endothelial cells form the and can extend/remodel the network of blood vessels for tissue growth and repair. The technique can contribute to the development of therapeutics for diseases-related to (region or area deprived of adequate oxygen supply) and inflammation.
The growth of new blood vessels at damaged sites is a pre-requisite for tissue regeneration. Bioengineers have locally treated such sites with pro-angiogenic molecules such as (VEGF) in a by injecting the molecule at target sites, or through . However, the molecule has unexpected side-effects influenced by off-target sites, inducing both angiogenesis as well as inflammation, which can even lead to . While researchers have reported a promising strategy to deliver with fibrin biomaterials, chronic issues related to pro-angiogenic molecular treatment require resolution using current approaches.
Engineering the channel network hydrogel
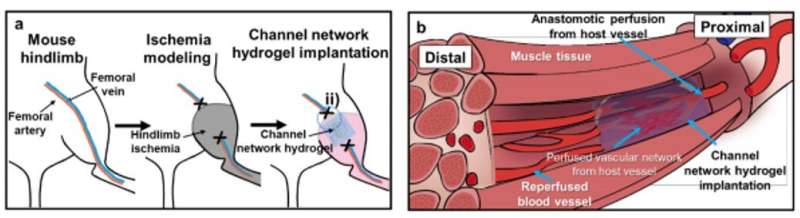
In this work, Lee et al. examined the induction of blood perfusion to damaged sites by implanting a hydrogel with microchannel networks to form functional vascular structures. They demonstrated the therapeutic effect in animal models and obtained a functional vascular structure with microchannels scaled for (EC) recovery during wound healing. The research team produced the 3-D channel network hydrogels using a three-step process using PNIPAM [poly (N-isopropylacrylamide)] to produce fibers embedded in a polydimethylsiloxane (PDMS) mode, followed by gelation cross-linking.
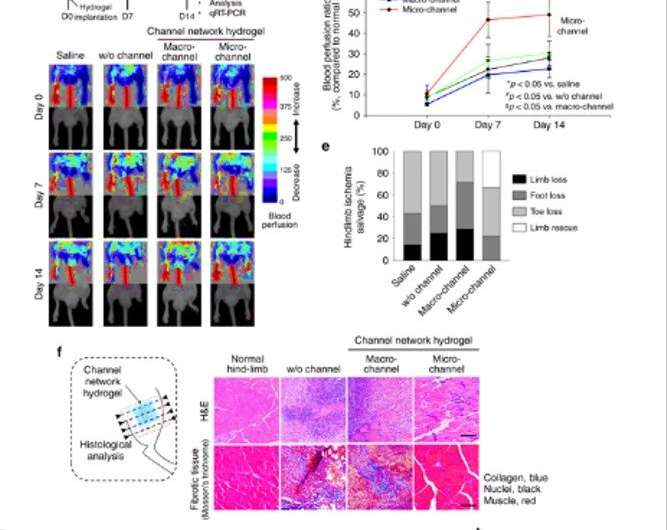
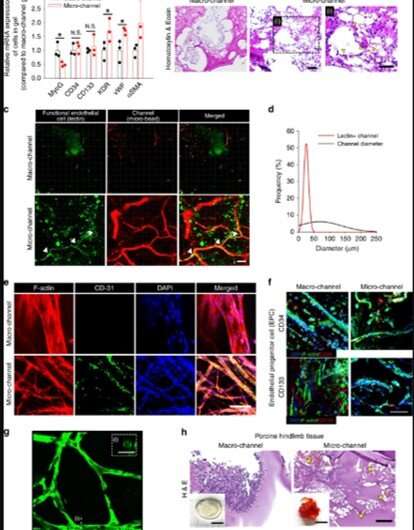
They generated void channel networks after dissolving fibers and perfusion washing. The scientists analysed the resulting channel structure and interconnectivity with fluorescence perfusion, followed by . The implantation process in an animal model allowed for blood perfusion from the proximal host vessels to microchannels and to the distal vessels to rescue ischemic limb tissue damage. For instance, in a mouse model of severe hindlimb ischemia, the research team quantified the degrees of blood perfusion using (LDPI) after implanting the test hydrogels. Thereafter, using they examined tissue samples from the distal site of the implant, 14 days after implantation. Groups with macro-sized channels or without channels showed invasion of massive inflammatory cells and severe fibrotic tissue formation. In contrast, the microchannel group showed significant restoration of muscle tissue to the level of a normal limb.
Investigating the primary mechanism of the microchannel hydrogel
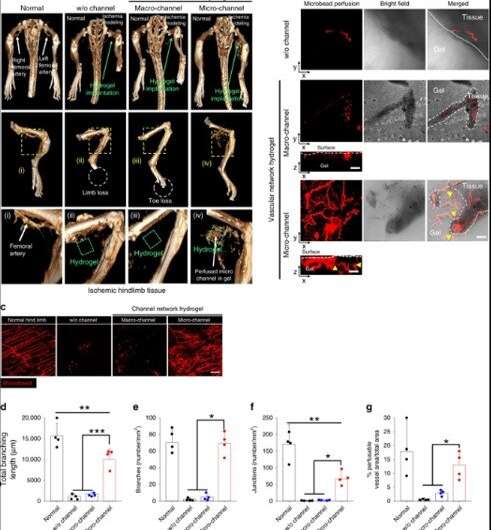
Lee et al. investigated the primary mechanism through which the severely damaged limb was rescued by the microchannel hydrogel using (micro-CT) and perfusion-based confocal imaging. They examined the perfusion connection between the host vessels and the microchannel network 14 days after implantation in a mouse model of severe hindlimb ischemia. As the blood vessel branching increased, the covered surface area increased to enhance the delivery of oxygen and nutrient to every corner of the tissue for improved wound repair.
When the scientists analysed the microchannel hydrogel implants using gene expression studies, they found that inflammatory cells infiltrated the device, which they verified via gene expression markers representating and . The degree of tubulogenic branching in the microchannel was greater than the control group to confirm the pro-angiogenic effects of the microchannel size. The team observed the effects of endothelialisation using gene and protein expression markers for endothelial cells (ECs) and observed significantly higher vascular lining cells in the microchannel group compared to the macro-channel. These results were supported by increased blood cell appearance and blood spreading in the microchannel group after implantation in an animal model of hindlimb ischemia.
Wound healing model and a non-ischemic in vivo model
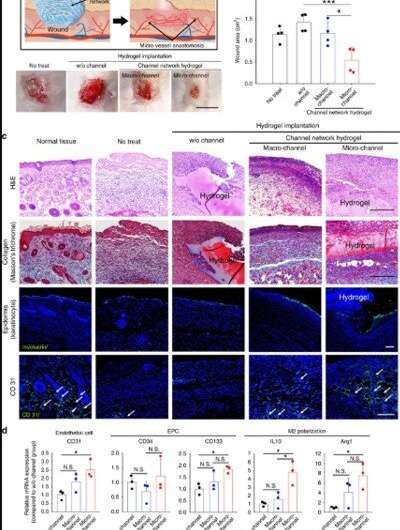
Lee et al. then examined the versatility of the microchannel-network hydrogel, by implanting it into another model of ischemic damage such as the well-established . Similar to the model of hindlimb ischemia, the neighboring vessels grew into the hydrogel to undergo perfusion connection with the microchannel network. Implantation of the microchannel group similarly accelerated the process of wound closure and the scientists supported the results with histological and molecular marker investigations that indicated robust tissue formation, richer collagen content and accelerated epidermis formation.
In addition to hindlimb ischemia and wound closure models, Lee et al. employed three other in vivo models to determine the mechanisms of ischemia and neighboring blood vessels. In this instance, they did not subject these models to ischemia. The results highlighted the function of ischemic conditions, as they assisted the microchannel-effect most effectively during vessel infiltration by assuming a mechanistic role in the setup.
In this way, Jung Bok Lee and colleagues developed an implantable hydrogel with a perfusable microchannel network to treat ischemic/inflammatory disease by promoting angiogenesis and upregulating macrophage/monocyte infiltration. The study can significantly contribute to the field of regenerative medicine in biotechnology to vascularize damaged tissues or implantable scaffolds. The work was based on the idea of using a pre-engineered 3-D microchannel network for vascularization perfusion throughout the hydrogel implant via the growth of neighboring host vessels and a perfusion connection of microchannels.
The scientists programmed the regenerative function only with the microchannel structure without loading any cells or therapeutic molecules into the setup. The microchannel size and closed 3-D network crucially guided the regenerative mechanism in the gelatin hydrogel implant. The enzyme-/crosslinked gelatin hydrogel was also biocompatible, cheap and allowed easy loading of therapeutics or cells prior to gelation.
The easy and fast fabrication process with scalable production can be translated from the bench to the clinic. The regenerative effect of the microchannel-network hydrogel is highly promising and also applicable to (PAD) treatment in a clinical setting. Lee et al. will design the next steps of this system to accelerate perfusion connection to host vessels to enhance clinical translation of the hydrogel.
Written for you by our author —this article is the result of careful human work. We rely on readers like you to keep independent science journalism alive. If this reporting matters to you, please consider a (especially monthly). You'll get an ad-free account as a thank-you.
More information: 1. Jung Bok Lee et al. Microchannel network hydrogel induced ischemic blood perfusion connection, Nature Communications (2020).
2. P. Martin. Wound Healing—Aiming for Perfect Skin Regeneration, Science (2002).
3. J. Street et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover, Proceedings of the National Academy of Sciences (2002).
Journal information: Nature Communications , Science , Proceedings of the National Academy of Sciences
Provided by Science X Network
© 2020 Science X Network