November 2, 2020 feature
Single-shot 3-D wide-field fluorescence imaging with a computational miniature mesoscope

Thamarasee Jeewandara
contributing writer

The online feature cover photograph on Science Advances this week displays fluorescence imaging with a computational miniature (CM2). The technique of fluorescence imaging is an essential tool for biologists and neuroscientists; however, conventional microscopes and miniaturized microscopes (miniscopes) are constrained by limited —a measurement of the information capacity of an optical system, shallow depth of field and an inability to resolve three-dimensional (3-D) distributed emitters. To overcome existing limits, Yujia Xue and a team of researchers in electrical and computer engineering, biology, neurophotonics and biomedical engineering at Boston University, U.S., developed a light and compact mesoscope known as the computational miniature mesoscope (CM2).
The new platform integrated a microlens for imaging and an LED array for excitation within the same setup. The device performed single-shot 3-D imaging and facilitated a 10-fold field-of-view gain and a 100-fold depth-of-field improvement, compared to existing miniscopes. Xue et al. tested the device with fluorescent beads and fibers alongside phantom experiments to measure the effects of bulk scattering and background fluorescence. The team discusses the practicality of this mesoscope for broad applications in biomedicine and 3-D neural recording.
Advancing fluorescence microscopy
Fluorescence microscopy is a key technique in . Recent technological developments are aimed at to investigate individual neurons of only a few microns in size. For example, , and have begun to bridge this scale; however, the development of such imaging systems is limited by scale-dependent geometric aberrations of optical elements. The achievable field of view (FOV) is also limited by the system's shallow depth of field in many bioimaging applications. Researchers are also focused on miniaturizing the technology to allow in vivo imaging in freely behaving animals. For example, miniaturized microscopes known as have gained unprecedented access to neural signals, although the systems remain restricted by their optics, much like their fluorescence microscopy counterparts. Xue et al. therefore introduced and demonstrated a computational miniature microscope (CM2) with large-scale, 3-D fluorescence measurements on a compact, light-weight platform.
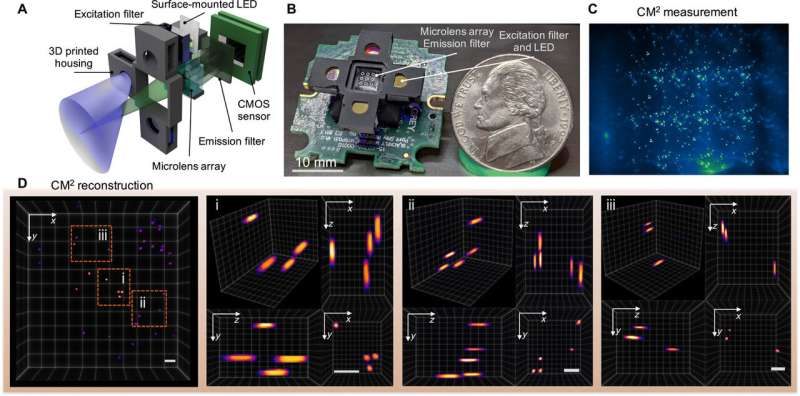
The mechanism-of-action of the computational miniature mesoscope (CM2)
The team used simple optics in the setup to accomplish space-bandwidth product (SBP) improvement and 3-D imaging capabilities without the need for mechanical scanning. The technique bypassed the physical limits of the integrated optics by jointly designing the hardware and the algorithm. The CM2 imaging method combined several different features of microscopic imaging, such as , and . In its mechanism of action, the microscope collected a single 2-D measurement using a microlens array (MLA) for subsequent computational reconstruction of the 3-D fluorescence distribution.
The CM2 used the microlens array as the sole imaging element and allowed the setup to overcome the field-of-view (FOV) limits imposed by the objective lens of conventional microscopes. The CM2 algorithm solved the 2-D-to-3-D deconvolution problem to . Xue et al. explained the principle of the CM2 single-shot 3-D imaging capability by drawing an analogy to (FDM). The team then quantified the achievable resolution of the CM2 by computing the 3-D (MTF) of the system and analyzing the lateral resolution.
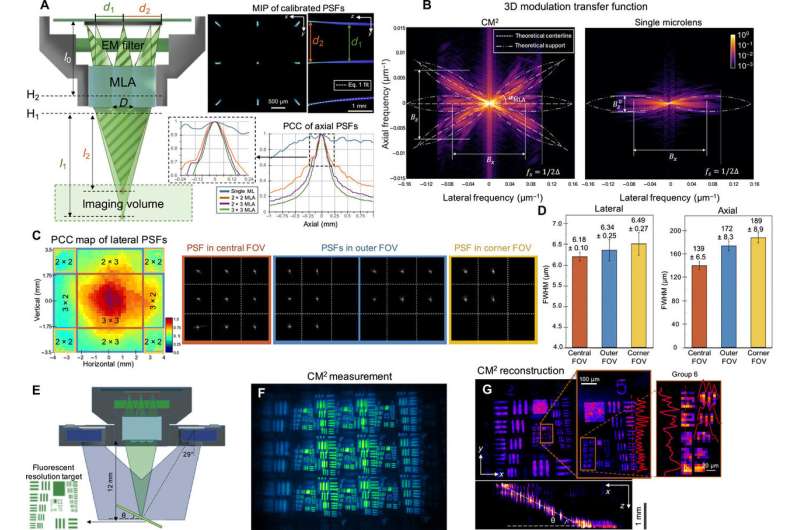
Proof-of-concept experiments
Xue et al. approximated the image formation of the CM2 setup by using a slice-wise model. They characterized the resolution and lateral shift variance of the setup prior to experimental imaging and imaged a fluorescent resolution target to validate the lateral resolution of the CM2. They validated the observations using measurements to find a good agreement between the simulations and the experiments. The new platform allowed the scientists to localize fluorescent emitters distributed across a large volume. They tested the performance of CM2 on samples with a feature size similar to a single neuron. During these experiments, the CM2 algorithm was tolerant of signal degradations such as reduced signal-to-noise ratios to enable high-quality, full-field-of-view reconstruction. The team compared the CM2 reconstruction and an axial stack acquired by an objective lens to demonstrate the accuracy of single-shot localization of individual particles.
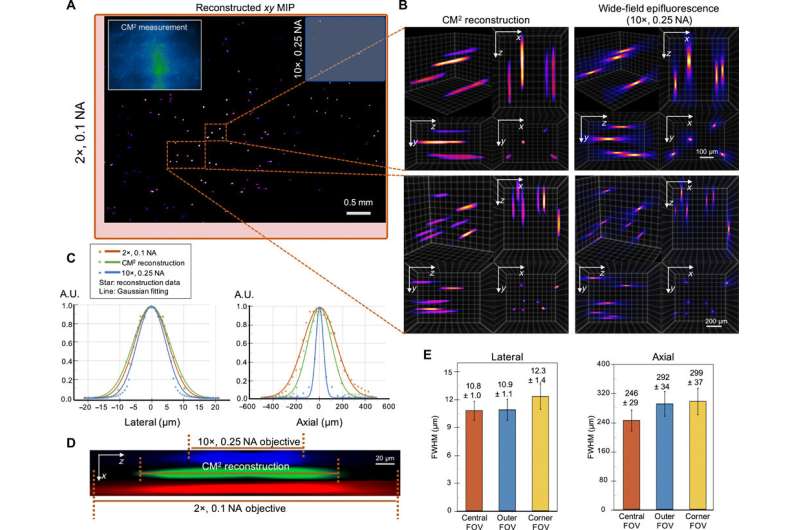
Experiments on fluorescent fibers on a curved surface and on controlled scattering phantoms.
The scientists next tested the ability to image complex volumetric fluorescent samples on fluorescent fibers spread on a 3-D printed curved surface, mimicking the surface profile of a mouse cortex, spanning a wide field-of-view and an extended depth. The algorithm accurately recovered the in-focus structures and solved for the 3-D object, while resolving most of the individual fibers. The team further conducted experiments on eight imaging phantoms to test the performance of CM2 under bulk scattering and strong background fluorescence. During the experiments, they seeded all phantoms with the same concentration of target fluorescent particles and credited the differences in reconstruction to bulk scattering and background fluorescence. The team then included 1.1-µm background fluorescent particles to mimic unresolvable fluorescent sources commonly seen on biological samples; such as in the brain. They quantified the scattering level for each phantom, performed 3-D reconstruction for each scattering phantom and performed all deconvolutions using the same computational setting. The estimated reconstruction depth range varied with surface variations present in each phantom.
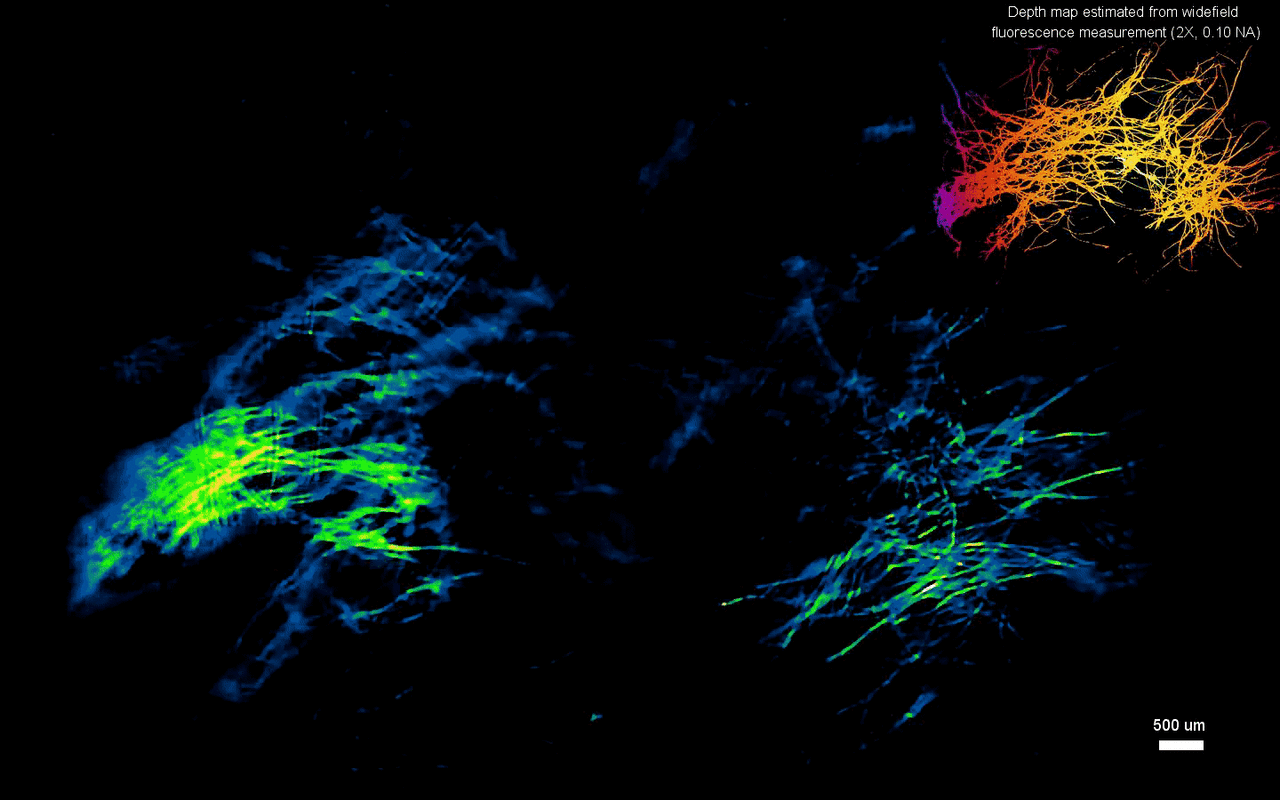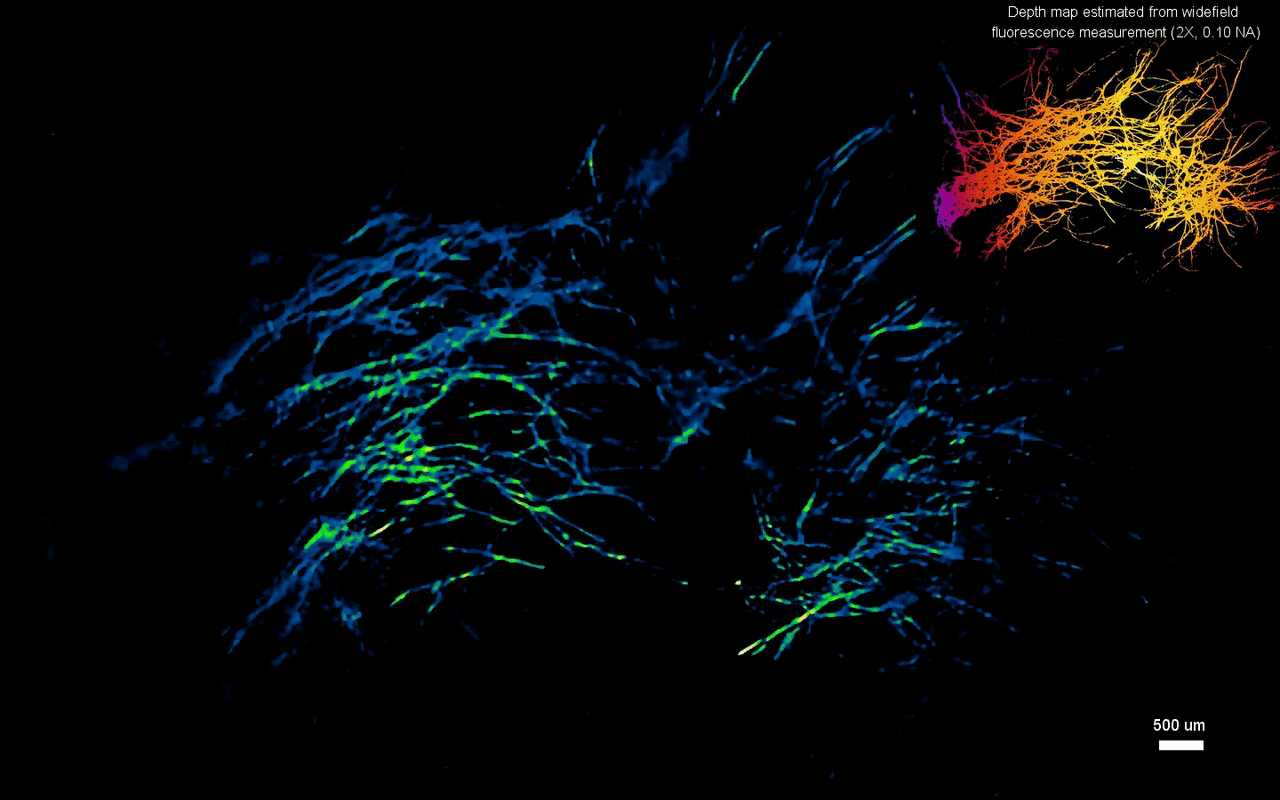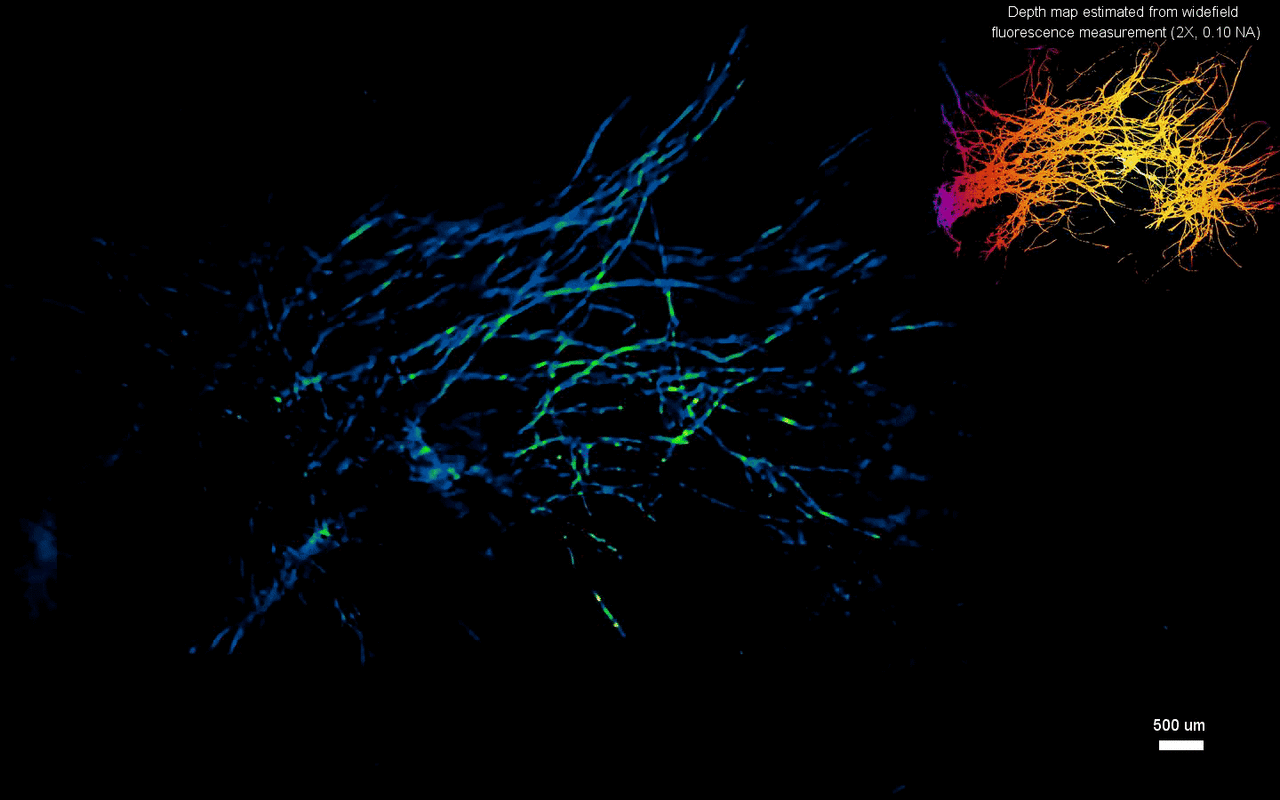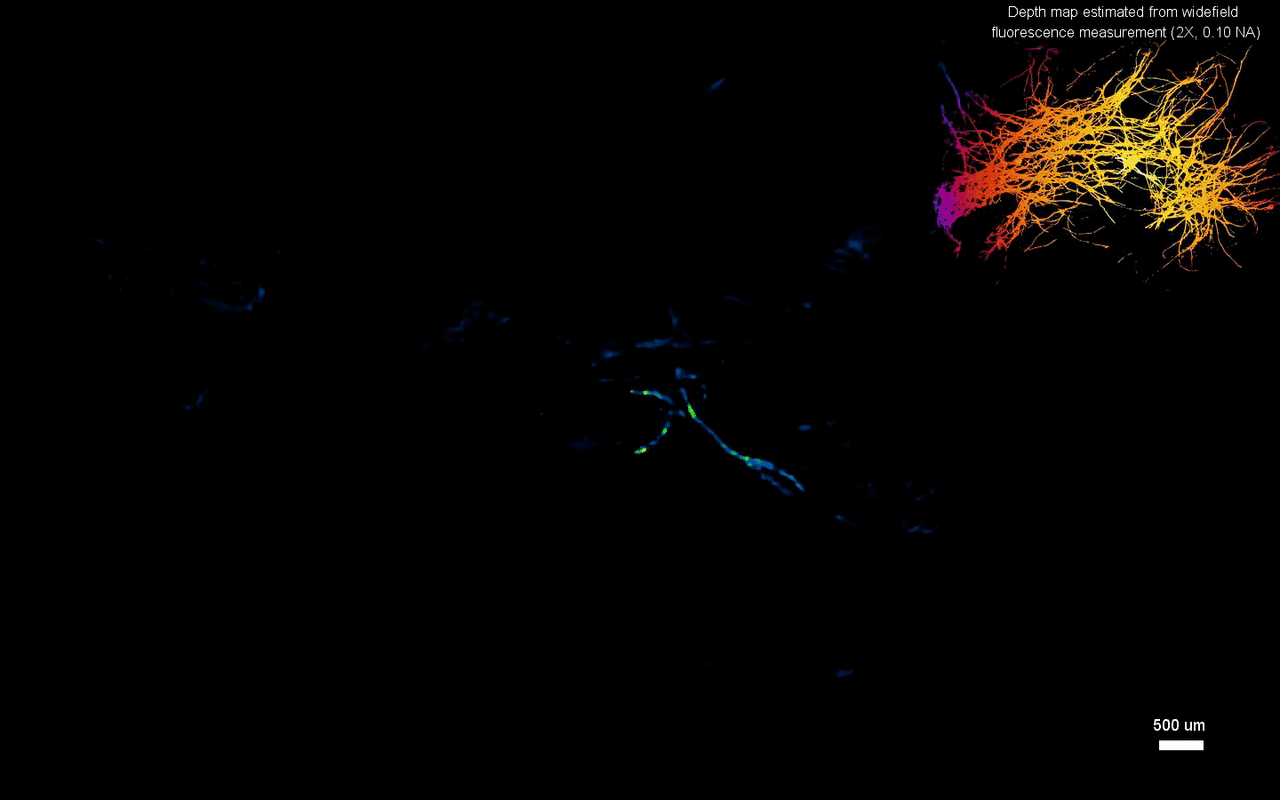
In this way, Yujia Xue and colleagues developed a new miniaturized fluorescence imaging system to allow single-shot mesoscopic 3-D imaging. The computational miniature (CM2) method integrated fluorescence imaging and the excitation modules on the same compact platform. The team presented the simulations and experiments to establish the mechanism of action and 3-D imaging capacity of the CM2. They simulated brain-wide imaging of vascular networks and the primary results were promising. The CM2 prototype is not yet comparable to head-mounted in vivo applications (on animal models) in neuroscience labs, although the team envision optimizing the device for full-cortical in vivo imaging in freely moving mice. The imaging device can be further improved with further developments in hardware and algorithms to open new and exciting opportunities within in vivo neural recording and biomedical applications.
Written for you by our author —this article is the result of careful human work. We rely on readers like you to keep independent science journalism alive. If this reporting matters to you, please consider a (especially monthly). You'll get an ad-free account as a thank-you.
More information: Yujia Xue et al. Single-shot 3D wide-field fluorescence imaging with a Computational Miniature Mesoscope, Science Advances (2020).
Jeff W Lichtman et al. Fluorescence microscopy, Nature Methods (2005). .
Sofroniew N. J. et al., A large field of view two-photon mesoscope with subcellular resolution for in vivo imaging, eLife,
Journal information: Science Advances , Nature Methods , eLife
© 2020 Science X Network




















