May 5, 2021 feature
Using flexible microparticles as drug carriers to shuttle nanoparticles to the vascular wall

Thamarasee Jeewandara
contributing writer

Drug carriers that target the must adhere to the endothelial vessel wall to achieve clinical stability. The particle size is a critical physical property to prescribe particle margination within biological blood flows and those conducted in-lab. While microparticles are optimal for , nanoparticles are better for intracellular delivery. In a new report now on Science Advances, Margaret B. Fish and a research team in chemical engineering, pharmacology and cardiovascular medicine and engineering at the University of Michigan, Ann Arbor U.S., tested flexible hydrogel particles as carriers to transport nanoparticles to a diseased vascular wall. Based on the microparticle , nanoparticle-loaded -based hydrogel microparticles delivered more than 50-nm nanoparticles to the vessel wall, when compared to freely injected nanoparticles to achieve more than 3000 percent increase in delivery. The work showed the benefit of optimizing the efficiency margination of microparticles to enhance transport of nanocarriers to the vascular wall.
Designing drug carriers
Drug carriers that target the vascular wall are usually made of polymeric particles engineered to adhere to sites of disease and accumulate via markers on the vessel wall for localized drug delivery. The physical properties of drug carriers can determine the circulation time, biodistribution, vascular adhesion and immune interactions. Efficient vascular wall adherence is vital for the accurate release of their drug payload to the diseased endothelium tissue. Although nanoparticles (20 to 80 nm in diameter) are , only less than 1 . Comparatively, microparticles with 2- to 3-micrometer-diameter appear to be . Fish et al. therefore examined the possibility of loading nanoparticles into vascular-targeted flexible microparticles to overcome the existing limits with free nanoparticles. Using nanoparticle-loaded hydrogel microparticles, the team showed the comparatively effective delivery of nanoparticles to the vascular wall. This outcome provides an avenue to increase the clinical use of nanoparticle drug carriers to treat common diseases.

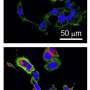
Developing and testing nanoparticle (NP)-loaded microparticles (MPs).
The scientists first fitted the hydrogel microparticle carriers with polymeric nanoparticles as cargo. To accomplish this, they chose (PS) NPs due to their uniform size distribution and the consistency of NP loads across different MP formulations. The team then tested parameters of particle adhesion to understand how rigid polystyrene nanoparticles with an affected the bulk modulus of the hydrogels. For this, they loaded the 50-nm polystyrene NPs into hard microparticles and noted no significant increase in the bulk shear modulus, as well as considerable flexibility. Then, Fish et al. tested the capacity of NP-loaded hydrogel microparticles to bind to an activated during human blood flow . Using the test assay, they quantified the number of nanoparticles and microparticles trafficked to the vessel wall. The team further studied the loaded hydrogel MPs relative to free NPs on a plate-reader. The results showed how drug carriers with higher NP loading delivered a significantly higher NP payload to the wall. Based on the constitution of diverse drug carrier prototypes, Fish et al. noted the 50 percent polyethylene glycol (PEG) constituting microparticles to have delivered the most nanoparticles. Compared to free NPs alone, the hydrogel microparticle delivery quantitatively achieved a 1550 percent increase in the number of nanoparticles to reach the vessel wall.

Nanoparticle (NP) vessel wall binding dynamics
Based on several control experiments, Fish et al. next confirmed how the difference between NPs delivered to vessel walls via MPs versus free NPs, did not merely rely on the free NPs binding to blood cells or being phagocytosed by . To accomplish this, they performed of blood samples collected after flow assays and found an insignificant number of leukocytes bound by NPs. In addition to that, when they incubated free NPs in static blood setups in the lab, only a very minimal number of blood cells bound NPs in static assays. The team therefore credited the low NP adhesion to be due to a failure to bind to the vessel wall, and not due to their clearance via , nor due to their non-specific binding to blood cells. They then conducted clinical tests to compare the adhesion of NP-loaded MPs vs. free 50 nm NPs in the of mice. They chose the mesentery with acute inflammation to visualize particle adhesion using . The hydrogel MPs were significantly more efficient at delivering 50 nm polystyrene nanoparticles to an inflamed mesentery in the biological model, compared to free NPs, regardless of the quantity of free NPs loaded.

Sustained adhesion of particles in time.
While nanoparticles are known to maintain longer circulation times when compared to micro-sized particles, it is assumed that 50 nm would outperform MPs across time. To understand this, the team assessed targeted particle binding duration by investigating and comparing three flexible particle types directly to the 50 nm polystyrene particles. They then captured particle adhesion in five distinct locations of the mesentery vein every five minutes for one hour. During the hour-long frame, the hydrogel NPs did not match or surpass the hydrogel MPs in targeted adhesion efficiency. The team next investigated a longer targeting window with an acute lung injury model and noted an extended presence of targeted flexible adhesion of the hydrogel MPs in vivo.

Outlook
In this way, Margaret B. Fish and colleagues showed how loading nanoparticles (NPs) into hydrogel microparticles (MPs) had excellent influence on improving the delivery of smaller NPs for a variety of clinical situations suited for targeted drug delivery. Due to their highly tuneable flexibility, the team could design the hydrogel carriers to ensure easy transport through the vasculature with low risk of vessel occlusion on binding, much like the native . When compared to free NPs, the soft hydrogel MPs offered significantly stronger and sustained adhesion, during all experiments. This work demonstrated a massive advantage of trafficking NPs to the vessel wall via the strategy of loading NPs into hydrogels and the outcome can be optimized for clinical applications across regenerative medicine and bioengineering.
Written for you by our author —this article is the result of careful human work. We rely on readers like you to keep independent science journalism alive. If this reporting matters to you, please consider a (especially monthly). You'll get an ad-free account as a thank-you.
More information: Fish M. B. et al. Deformable microparticles for shuttling nanoparticles to the vascular wall, Science Advances,
Nel A. E. et al. Understanding biophysicochemical interactions at the nano-bio interface. Nature Materials,
Tasciotti, E. et al. Mesoporous silicon particles as a multistage delivery system for imaging and therapeutic applications. Nature Nanotechnology,
Journal information: Nature Materials , Science Advances , Nature Nanotechnology
© 2021 Science X Network