May 28, 2021 feature
The hypothalamus predates the origin of vertebrates

Thamarasee Jeewandara
contributing writer
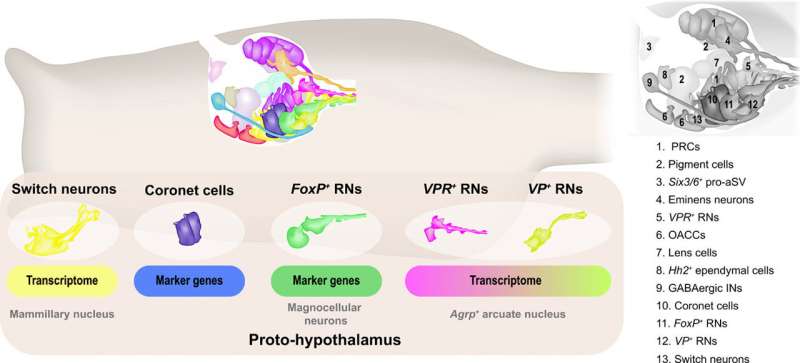
The hypothalamus is involved during the coordination of neuroendocrine functions in vertebrates and their evolutionary origin can be described using integrated transcriptome or connectome brain maps of swimming tadpoles of , also known as sea vase. These organisms serve as an approximation of their ancestral protovertebrate. The map included several cell types relative to different regions of the vertebrate hypothalamus, including the , arcuate nucleus and . These observations highlighted how the hypothalamus predates the evolution of the vertebrate brain. The neural crest and are key innovations that contributed to the evolution of the vertebrate head. However, less is known about the evolutionary origin of the crown and summit of the vertebrate brain. In a new study now on Science Advances, Laurence A. Lemaire and a research team in molecular biology and integrative genomics at the Princeton University, New Jersey, U.S., used an extensive single-cell transcriptome fate map of the to characterize the neural cell types comprising its .
The hypothalamus
The sensory vesicle of the Ciona tadpole contains and is primarily responsible to relay sensory information including light, gravity and mechanical cues to the motor ganglion that controls the tadpole tail. The central nervous system (CNS) of the Ciona has facilitated lineage maps to allow the of a chordate. In an attempt to include the synaptic connectome, Lemaire et al. studied the evolutionary origins of the vertebrate brain, specifically the hypothalamus. The hypothalamus has ancient origins and forms an ancient region of the vertebrate brain. The construct is found across all vertebrates including fish to humans, the hypothalamus controls homeostasis, metabolism and reproductive functions through intricate interconnecting neural circuits. In this work, Lemaire et al. propose the major function of the Ciona proto-hypothalamus to be to trigger the onset of metamorphosis of the tadpole species.
Brain map
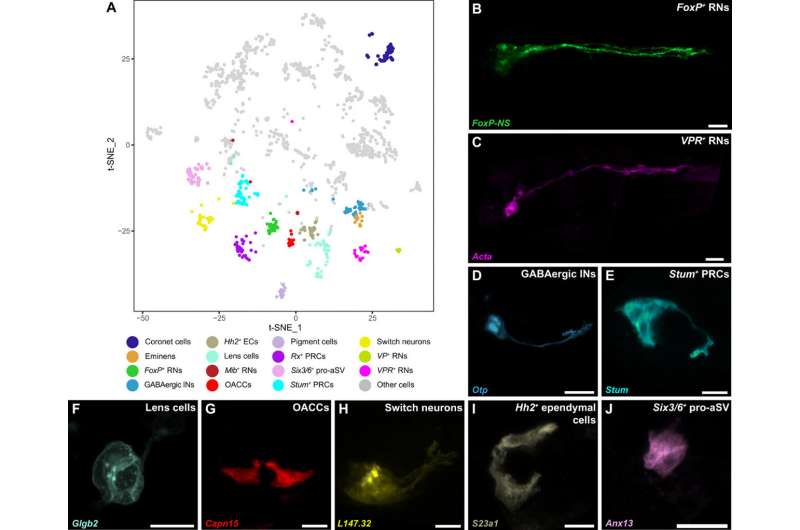
The scientists conducted of the Ciona intestinalis embryogenesis from gastrulation to swimming larvae, to identify 40 of the CNS (central nervous system) and peripheral nervous system. Lemaire et al. mapped each of the neural cell types comprising the simple brain of the tadpoles also known as their sensory vesicles. Based on the studies, they identified 15 different neural cell types including previously identified coronet cells, and pigment cells. Using neural-specific reporter genes, the team identified a range of (RNs) including those that expressed (FoxP+), as well as others expressing the vasopressin receptor (VPR+). The FoxP+ relay neurons were while the VPR+ relay neurons were . The researchers identified an underappreciated population of putative mechanosensory neurons, which they renamed 'switch neurons," corresponding to ciliated brain interneurons in the connectome map of the CNS of tadpoles.
![Coronet-associated circuit in swimming tadpoles. (A and B) Expression of reporter genes for melanopsin [(A), green] or pinopsin [(B), green] and a Ptf1a reporter gene (red) in coronet cells. (C) Switch neurons (L147.32 reporter; magenta) are closely associated with coronet cells (Ptf1a reporter; cyan) and the otolith (βγ-crystallin reporter; yellow) without touching the latter. (D) Coexpression of Ptf1a and Adra2 reporter genes (red and green, respectively) also show close associations of switch neurons and coronet cells. (E to E″) Higher-magnification views of the reporter genes shown in (D). (E) is a z-projection, (E′) y-projection, and (E″) x-projection, which highlights extensive cell-cell contacts between switch neurons and coronet cells. (F) Expression of a FoxP+ reporter gene (green) shows close contact of FoxP+ RNs with coronet cells (Ptf1a reporter; red). (G to G″) Higher-magnification views of the reporter genes shown in (F), corresponding to z-, y-, and x-projections, respectively. (H) t-SNE plot of Pkd2l expression across the nervous system (n = 2021 cells). The yellow dotted circle and arrow indicate the switch neurons, while the green dotted circle and arrow point to the FoxP+ RNs. (I) Immunostaining for acetylated tubulin (red) reveals cilia in switch neurons that were labeled with C2.478 reporter (green). All reporter assays were analyzed at the larva stage. Reporter genes code for membrane fluorescent proteins and their description as well as the number of replicates are provided in table S1. White dashed lines indicate the outline of the trunk regions of tadpoles, while yellow dotted and dashed lines identify the pigment of the ocellus and the otolith, respectively. Scale bars, 10 μm (A, B, E, and G) and 20 μm (C, D, F, and I). Credit: Science Advances, doi: 10.1126/sciadv.abf7452 The hypothalamus predates the origin of vertebrates](https://scx1.b-cdn.net/csz/news/800a/2021/the-hypothalamus-preda-1.jpg)
Neuronal circuit
Scientists had previously identified similarities of with the vertebrate hypothalamus since they released dopamine to express diverse neuropeptides including neurotensin-like B and (Gnrh). However, these cells were previously described to share morphological similarities with the coronet cells or a region of the hypothalamus present in non-tropical fish. The fish coronet cells also expressed and detected a short wavelength light associated with seasonal lengthening of daylight to trigger reproduction by releasing a thyroid-stimulating hormone, followed by the secretion of Gnrh (gonadotropin-releasing hormone) as in . The work showed how coronet cells functioned as light-sensing sensory cells relative to their dopaminergic and neurosecretory activities. The coronet cells also interacted with adjacent neurons such as and FoxP+ relay neurons. On the basis of their anatomical position, the switch neurons corresponded to ciliated brain interneurons. On the basis of cell-cell associations, the VPR+ relay neurons also received inputs from switch neurons.
Mechanosensory switch neurons
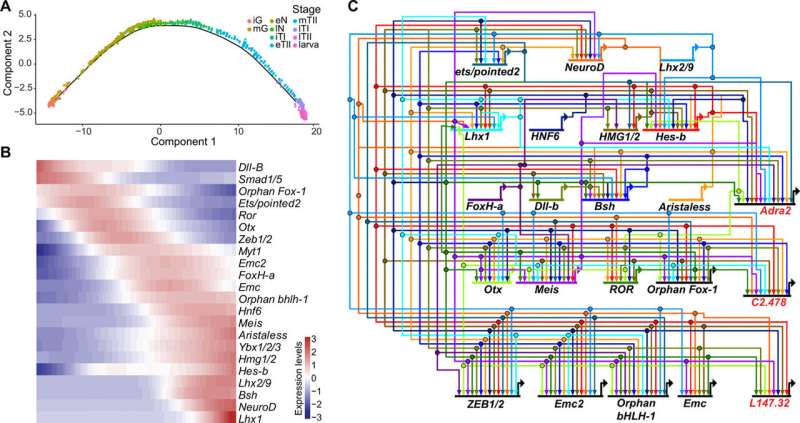
Previous studies had shown coronet cells to be a central sensory node for associated neurons and FoxP+ relay neurons. While currently exists on the networks that underly the specifications of coronet cells, not much is known about the development of switch neurons or FoxP+ relay neurons. Lemaire et al. therefore focused on switch neurons due to their roles as specialized mechanosensory cell types in vertebrates, including the cerebrospinal fluid containing neurons present along the central canal and the ventricular cavities of the brain . Additionally, not much is also known about the development or function of vertebrate (CSF-cNs). To understand their ontogeny, Lemaire et al. created a provisional gene regulatory network for switch neurons, using previously published methods. The team identified transcriptome trajectories and temporal cascades of genes encoding transcription factors in the cell lineages to form switch neurons. They represented the resulting interconnections as a provisional gene network. As proof of concept, they used single-cell RNA sequencing (scRNA-seq) assays to understand the cell types that are transformed into switch neurons after the mis-expression of a gene, to test the authenticity of the network.
Orthology maps of mouse hypothalamus and Ciona nervous system
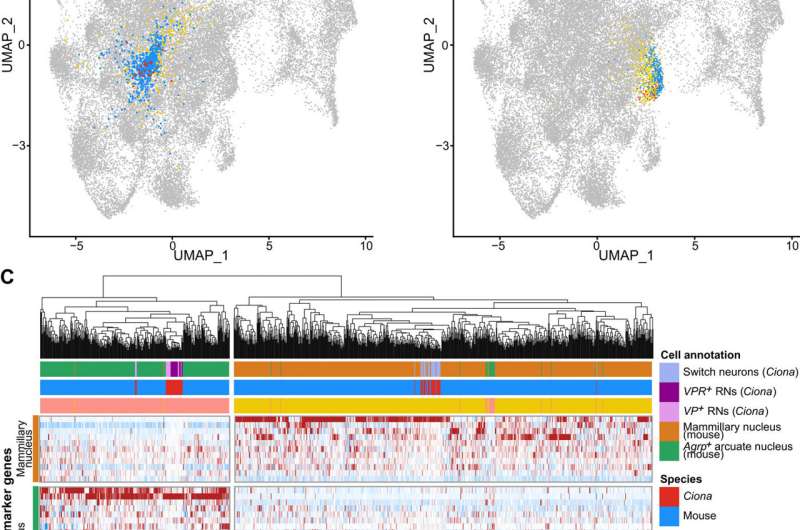
The team formed a putative sensory circuit featuring coronet cells as a central node, in association with switch mechanosensory neurons and the relay neurons. Existing studies alongside the current demonstration of coronet cells that express melanopsin and pinopsin provided considerable evidence for the homology with the vertebrate hypothalamus. To test if the relay neurons and the associated switch might share homology with the hypothalamus, Lemaire et al. for each of the 40 neural cell types comprising the Ciona nervous system and compared them with the transcriptome maps of the mouse hypothalamus. The studies identified two Ciona lineages that matched two different clusters of mouse hypothalamic cells. The combined comparative transcriptome analyses suggested the coronet-associated neural circuit to contain multiple cell types relative to different regions of the mouse hypothalamus.
Outlook
In this way, Laurence A. Lemaire and colleagues identified 15 different cell types in the sensory vesicle of Ciona larvae, while the connectome map identified 31 cell types. The team credited this disparity to reflect the different methods of classification. For instance, they noted how a single cell type based on intrinsic genetic properties could acquire distinctive behaviors through associations with different neurons. The scientists described five different types of relay neurons based on the transcriptome trajectories and profiles, while the connectome map identified 11 such neurons relative to synaptic inputs. The outcomes suggested the simple brain morphology of Ciona to contain a complex proto-hypothalamus with a role during the onset of metamorphosis in the tadpoles. Regardless of the intended function, this work indicates the evidence of multiple hypothalamic cell types in Ciona to suggest an unexpectedly sophisticated blueprint for the evolution of the complex vertebrate brain.
Written for you by our author —this article is the result of careful human work. We rely on readers like you to keep independent science journalism alive. If this reporting matters to you, please consider a (especially monthly). You'll get an ad-free account as a thank-you.
More information: Lemaire A. L. et al. The hypothalamus predates the origin of vertebrates, Science Advances, 10.1126/sciadv.abf7452
Abitua P. B. et al. Identification of a rudimentary neural crest in a non-vertebrate chordate. Nature,
Kindt K. S. et al. Caenorhabditis elegans TRPA-1 functions in mechanosensation. Nature Neuroscience,
Journal information: Science Advances , Nature Neuroscience , Nature
© 2021 Science X Network