Exploring high selective catalysts via fabrication of oxygen vacancy on TiO2
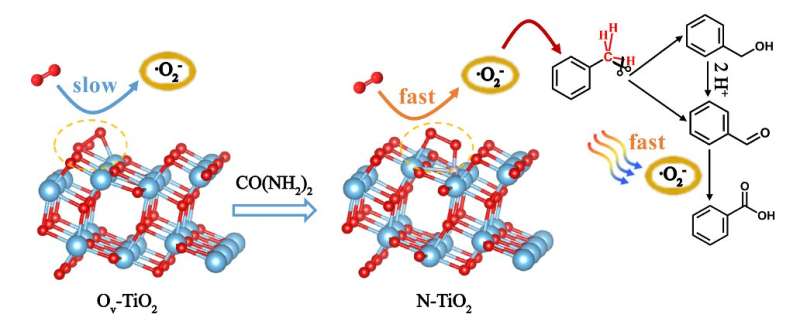
Oxygen vacancy (Ov) significantly influences the oxidation process through oxygen adsorption and activation. Element doping can fabricate oxygen vacancy on titanium dioxide (TiO2), but the effects of the dopants on the oxidation reaction over oxygen vacancy remain unclear.
A research team from the Research Center for Eco-Environmental Sciences of the Chinese Academy of Sciences has recently fabricated oxygen vacancy by doping nitrogen into anatase TiO2. Their results were published in Cell Reports Â鶹ÒùÔºical Science.
To fabricate oxygen vacancy with different structures, the researchers doped nitrogen (N) and boron (B) into anatase TiO2 (N-TiO2 and B-TiO2). Both N-–T¾±3+–Ov and Ti3+–Ov were observed in N-TiO2, but only Ti3+–Ov in TiO2 and B-TiO2. The results showed that N-–T¾±3+–Ov is more reactive than Ti3+–Ov in O2 activation.
In addition, the N-–T¾±3+–Ov active sites formed in N-TiO2 significantly enhance the thermal yield and selectivity of the oxidation of the primary C–H bonds in toluene.
The adsorption and activation of O2 are the rate-limiting step in the selective oxidation of primary C–H bonds in toluene. N-–T¾±3+–Ov as electron donors contributed to a rapid formation of superoxygen species (·O2-), which demonstrated to be active oxygen for primary C–H bond oxidation.
The fabrication of N-–T¾±3+–Ov sites opens a new avenue for dopants to improve the oxygen vacancy reactivity and enhance the primary C–H oxidation selectivity.
More information: Cheng Chen et al, Electron-donating N-–T¾±3+–Ov interfacial sites with high selectivity for the oxidation of primary C–H bonds, Cell Reports Â鶹ÒùÔºical Science (2022).
Journal information: Cell Reports Â鶹ÒùÔºical Science
Provided by Chinese Academy of Sciences