Using a bowl-structured active site design to break the scaling relations for nitrogen to ammonia conversion
The N2-to-NH3 conversion is a fundamental chemical process to supply nitrogen for modern industry and agriculture. Enormous efforts have been made since the invention of Haber-Bosch process, but it is still a challenging task to deliver N2-to-NH3 conversion at mild conditions.
A general issue arises from the scaling relations, which impose an apparent contradiction between the capabilities of a catalyst to activate N2 and to release NH3. This results in a volcano curve of the catalytic activity for the N2-to-NH3 conversion, and thus poses a limit on the catalytic performance by the optimal catalyst design.
This is the Sabatier principle for the catalyst design, which states that the adsorption of a relevant intermediate on the optimal catalyst should be neither too strong nor too weak. In other words, the optimal catalyst should be a compromise. Therefore, it is appealing to identify and elucidate the catalytic processes that are not dictated by the scaling relations, in order to design highly efficient heterogeneous catalysts beyond the compromise.
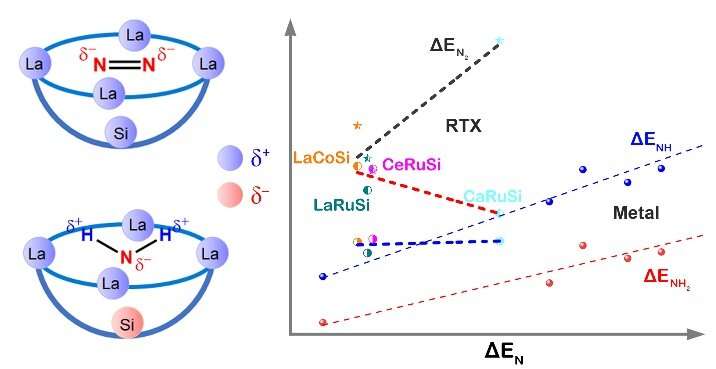
Recently, a research team led by Prof. Hai Xiao at Tsinghua University, China investigated the thermocatalytic mechanisms for N2-to-NH3 conversion on the intermetallic electride LaRuSi by first-principles calculations. They find that a bowl active site, composed of surface La cations and negatively charged subsurface Si atom originated from the electride nature, is key to the efficient catalysis of N2-to-NH3 conversion.
The electrostatic and orbital interactions between this bowl active site and reaction intermediates greatly enhance the N2 activation that results in negatively charged N2 for facile cleavage of NN bond, while destabilize the adsorptions of NHx (x = 1, 2, 3) species that contain positively charged H atoms, which facilitates the desorption of the final NH3 product. It is this particular bowl active site composed of f-block La cations and electride Si anion that breaks the scaling relations for highly efficient N2-to-NH3 conversion.
By comparison with other intermetallic electride catalysts isostructural to LaRuSi, they explicitly confirm the breaking of scaling relations between the adsorptions of NHx species and that of N. The adaptive electrostatic interactions exerted by the bowl active site play the key role in breaking the scaling relations for N2-to-NH3 conversion.
They also identify the possible presence of similar bowl active sites in other types of highly efficient heterogeneous catalysts. Thus, they propose this bowl active site with adaptive electrostatic interactions as a design concept, which sheds novel insights on the design of highly efficient heterogeneous catalysts for the N2鈥搕辞鈥揘贬3 conversion, as well as other catalytic reactions that are dictated by the scaling relations.
The results were published in Chinese Journal of Catalysis.
More information: Ya-Fei Jiang et al, Breaking the scaling relations for efficient N2-to-NH3 conversion by a bowl active site design: Insight from LaRuSi and isostructural electrides, Chinese Journal of Catalysis (2022).
Provided by Chinese Academy of Sciences