October 7, 2022 report
Creating a mouse embryo from stem cells to learn more about the mammalian development process

Bob Yirka
news contributor
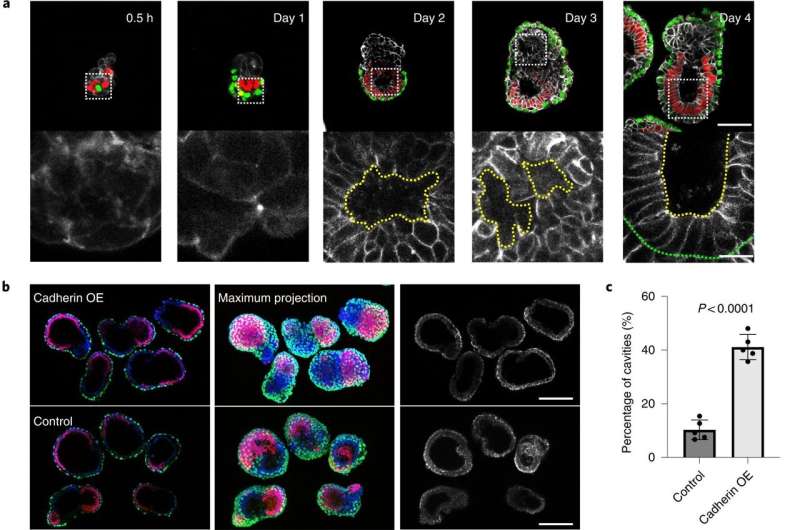
A team of researchers at the California Institute of Technology, working with one colleague from The Francis Crick Institute and another from the University of Cambridge, both in the U.K., has developed a way to grow mouse embryos without using mouse eggs or sperm to learn more about early mammalian development. In their paper published in the journal Nature Cell Biology, the group describes using several types of stem cells to grow mouse embryos.
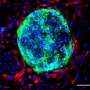
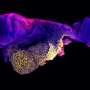
Prior research has shown that mammalian embryos differentiate into different types of cell masses as they develop. Researchers have also found that stem cells are involved in the processes but the mechanisms responsible are still unknown. In this new effort, the researchers used three different kinds of stem cells to grow a mouse embryo that matured to the point of having a beating heart and the beginnings of a brain.
To create such embryos, the researchers first studied communications between stem cell groups in naturally developing mouse embryos. They learned to recognize the elements that went into such communications and the means by which it was carried out. In essence, they "deciphered the code." They then isolated three main types of stem cells that made up the cell masses in early embryo development: pluripotent, which eventually grow to become body tissue, and two other types that grew to become the amnionic sac and placenta. They also noted the quantities of each type of stem cell.
The next step was to attempt to create a mouse embryo from scratch using the three types of stem cells in a lab setting. With careful tending, the researchers grew an embryo that matured enough to allow for study of its development.
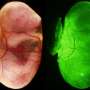
To test further, the researchers repeated the procedure but added genetically engineered cells to see how it impacted maturation of the embryo. They found they could replicate some of the same brain development issues that have been seen in human embryos. They suggest their work could also help explain what goes wrong when mice (or people) miscarry.
Written for you by our author —this article is the result of careful human work. We rely on readers like you to keep independent science journalism alive. If this reporting matters to you, please consider a (especially monthly). You'll get an ad-free account as a thank-you.
More information: Min Bao et al, Stem cell-derived synthetic embryos self-assemble by exploiting cadherin codes and cortical tension, Nature Cell Biology (2022).
Journal information: Nature Cell Biology
© 2022 Science X Network