A novel catalyst for efficient oxidation of inactive alkanes
A discovery in the field of catalysis has emerged from the laboratories of Professor Jaeheung Cho and his team in the Department of Chemistry at UNIST. Their pioneering work has led to the development of a copper(II)鈥揳lkylperoxo complex that could revolutionize the realms of synthetic chemistry and industrial applications. is published in ACS Catalysis.
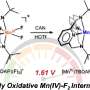
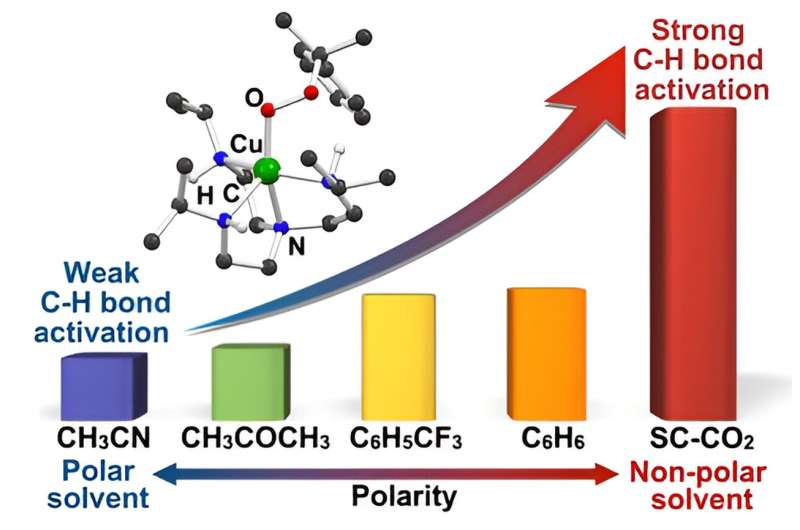
The key to this innovative catalyst lies in its remarkable ability to decompose strong carbon-hydrogen bonds (C鈥揌 bonds) through harnessing specific solvents. By precisely manipulating the solvent environment, the researchers have uncovered the exceptional reactivity of their copper(II)鈥揳lkylperoxo complex.
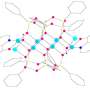
Through a meticulously designed series of experiments, the research team successfully synthesized the copper(II)鈥揳lkylperoxo complex and subjected it to supercritical carbon dioxide (厂颁鈥揅翱2), a fluid state of carbon dioxide that exhibit both gas and liquid properties simultaneously. This novel approach resulted in the most reactive metal鈥揳lkylperoxo peroxide compound to date.
Professor Cho said, "Our comprehensive analysis of oxidation reactions and advanced theoretical calculations have introduced a new era in oxidation catalysis utilizing copper(II)鈥揳lkylperoxo as a catalyst."
Of particular significance is the oxidation of unactivated alkanes, like methane and ethane, traditionally known for their stability and energy-intensive oxidation processes. By tailoring the copper(II)鈥揳lkylperoxo complex composition, the researchers achieved selective oxidation of unactivated alkanes, a pivotal advancement in catalytic science. Moreover, the team's exploration of various solvents confirmed the unprecedented ability of their catalyst to break down resilient C鈭扝 bonds.
Yuri Lee, the first author of the study, said, "Our research signifies a milestone in reactivity manipulation through solvent engineering within copper(II)鈥揳lkylperoxo species."
Professor Cho said, "Our work not only showcases the exceptional oxidation capabilities of copper(II)鈥揳lkylperoxo species, but also elucidates their solvent-dependent reactivity, laying the foundation for cutting-edge metal catalysts in various scientific domains."
The research team anticipates that this transformative research not only propels the boundaries of synthetic chemistry, but also holds immense promise for environmental and industrial applications, heralding a new era of catalytic excellence and sustainable technology.
More information: Yuri Lee et al, Influence of Solvents on Catalytic C鈥揌 Bond Oxidation by a Copper(II)鈥揂lkylperoxo Complex, ACS Catalysis (2024).
Journal information: ACS Catalysis