Researchers discover how nature repurposes ammonium transporters as receptors
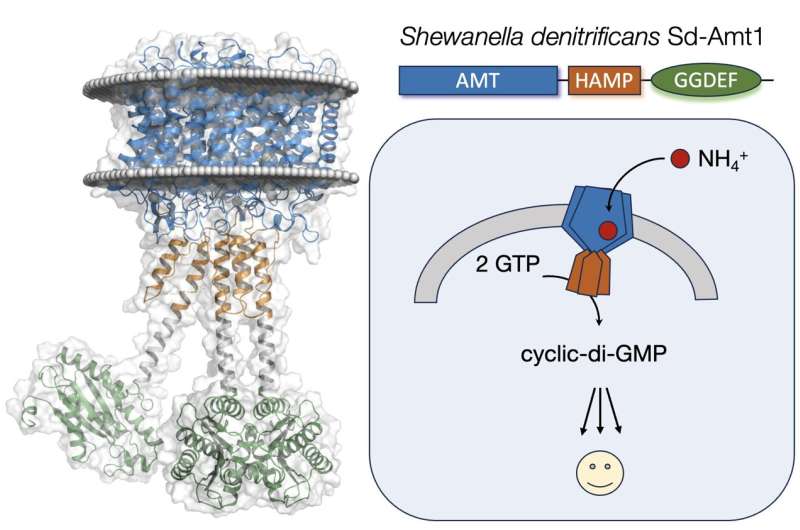
A team led by Freiburg biochemist Prof. Dr. Susana Andrade has characterized a new membrane protein that allows microorganisms to repurpose ammonium transporters (Amts) as receptors.
Ammonium transporters clearly distinguish between ammonium, potassium and water. This property has certainly contributed to their conversion into ammonium receptors in the course of evolution.
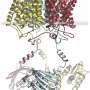
The newly discovered protein Sd-Amt1 utilizes ammonium cations as extracellular signals to increase the cytoplasmic level of the secondary messenger cyclic-di-GMP. It consists of a membrane-integral ammonium receptor domain linked to a cytoplasmic diguanylate cyclase transducer module.
The first authors of the study are Freiburg biochemists Dr. Tobias Pflüger and Dr. Mathias Gschell from the University of Freiburg. The results have been in the journal Science Advances.
The research team's work combines modern biochemical, biophysical and bioinformatics tools to integrate high-resolution structural and functional data. They enabled the researchers to reveal the molecular details of ammonium-triggered signal binding, receptor activation and transducer modulation.
In collaboration with the bioinformatics group of Prof. Dr. Stefan Günther from the Institute of Pharmaceutical Bioinformatics at the University of Freiburg, they were also able to show the extent to which the signal binding motif is conserved between prokaryotic and eukaryotic organisms. The work thus also provides a signature motif for identifying other ammonium receptors.
More information: Tobias Pflüger et al, How sensor Amt-like proteins integrate ammonium signals, Science Advances (2024).
Journal information: Science Advances
Provided by University of Freiburg