Dissecting caspase-2-mediated cell death: From intrinsic PIDDosome activation to chemical modulation

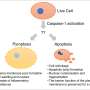
Caspase-2, an initiator caspase, plays a critical role in programmed cell death in response to certain cellular stresses. Its activation is facilitated by the PIDDosome, a multi-protein complex that assembles under conditions of genotoxic stress. Despite caspase-2's importance, the mechanisms through which it triggers cell death have remained unclear due to its inability to directly activate effector caspases. Recent research addresses this gap by demonstrating that caspase-2 directly processes BID, initiating the mitochondrial pathway of apoptosis.
Moreover, the team discovered HUHS015, a compound that specifically activates caspase-2-mediated apoptosis independently of the PIDDosome. The study also highlights the structural differences between human and mouse caspase-2 that confer selectivity for human caspase-2 by HUHS015 derivatives.
Key findings from the study include:
- BID is essential for PIDDosome-induced caspase-2-mediated apoptosis. Caspase-2 directly cleaves BID, which then signals the mitochondrial pathway leading to apoptosis.
- Identification of HUHS015 as a specific activator of caspase-2. This compound activates caspase-2-mediated apoptosis independently of the PIDDosome, requiring BID for its action.
- Structural specificity of caspase-2 agonists. HUHS015 and its derivatives selectively target human caspase-2 due to two critical amino acid differences in the interdomain linker region compared to mouse caspase-2.
The findings elucidate the role of caspase-2 in apoptosis, particularly in the context of genotoxic stress and its connection to BID. The discovery of HUHS015 and its derivatives as potent activators of human caspase-2 provides a novel approach to understanding the physiological functions of caspase-2-mediated cell death. These compounds represent promising tools for further exploration of caspase-2 biology and may serve as a foundation for the development of small molecule drugs targeting diseases related to dysregulated apoptosis.
The work titled "Dissecting caspase-2-mediated cell death: from intrinsic PIDDosome activation to chemical modulation" was on Protein & Cell.
More information: Mengxue Zeng et al, Dissecting caspase-2-mediated cell death: from intrinsic PIDDosome activation to chemical modulation, Protein & Cell (2024).
Provided by Higher Education Press