Light-responsive gene regulation at the mRNA level

Researchers at the University of Bayreuth have established a new optogenetic approach that can control the bacterial production of proteins at the mRNA level using blue light. The new system gates the activation of the genetic substance particularly effectively and thus surpasses previous approaches. It provides new tools for basic research and biotechnology.
Optogenetics refers to the regulation of biological processes by light, for example, gene expression, which is the activation of specific genes. Optogenetics therefore offers a promising approach for biotechnology and "theranostics"—a combination of therapy and diagnostics. It makes it possible to control the production of proteins in cells.
In addition to providing tools to further basic research and for biotechnological applications, the findings of the Bayreuth researchers also bring significant progress for the general control of RNA-based cellular processes by light. The results can be used to build genetic circuits that control the activity and state of RNAs within bacteria and mammalian cells.
The findings are in the journal Nucleic Acids Research.
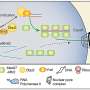
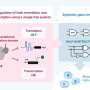
Optogenetic methods have so far been based almost exclusively on activating the transcription of DNA into mRNA (messenger RNA). However, the study by the Photobiochemistry group at the University of Bayreuth goes one step further: The researchers established a new optogenetic approach, dubbed riboptoregulator, to activate bacterial gene expression at the mRNA level using blue light.
The advantages of controlling gene expression at the mRNA level include the speed of the response, modularity and combinability with other genetic circuits.
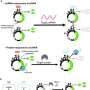
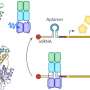
The team led by Prof. Dr. Andreas Möglich used the photoreceptor PAL, which they already discovered a few years ago. Upon activation with blue light, PAL can bind specific RNA structures and release a blockade at the so-called translation initiation region. Ribosomes, which are responsible for translating the mRNA into proteins, dock onto this region. Once the blockade has been released by PAL, the mRNA can be translated.
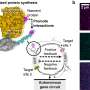
"We exploited the modularity of the riboptoregulator module and combined it with other genetic circuits to establish the new pAurora2 system. The resultant, integrated setup controls bacterial gene expression in response to blue light in a particularly stringent manner and surpasses previous approaches," says Möglich.
The pAurora2 system is so efficient because it promotes gene expression at two points. First, pAurora2 releases the blockade of translation of the target gene on the mRNA strand, and second, the system suppresses the expression of a translation repressor. In this way, the expression of a target gene can be boosted more than 1,000-fold.
"This regulation at the RNA level brings many advantages that in the future can be used for modern applications of light-regulated bacterial gene expression in theranostics, biotechnology or materials science," says Dr. Américo Ranzani, first author of the study and a postdoc in the Photobiochemistry research group at the time it was carried out.
More information: Américo T Ranzani et al, Induction of bacterial expression at the mRNA level by light, Nucleic Acids Research (2024).
Journal information: Nucleic Acids Research
Provided by Bayreuth University