December 2, 2024 feature
Scientists develop self-sustained protein transport and tissue assembly in artificial cells

Tejasri Gururaj
contributing writer

In a new Nature Communications , scientists have developed a novel method for artificial cells to interact with their external environment without the need for complex modification processes.
This method could open new frontiers in tissue engineering, drug delivery, and cell processes.
Biological cells are protected by a membrane, made of phospholipids, which modulates interactions with the outside environment. Recreating this in artificial cells is challenging, requiring manual external modification of the membrane.
This is particularly true for protein translocation or movement across the membrane. The present study addresses this problem by developing a method in which artificial cells modify their own membrane.
Â鶹ÒùÔº spoke to two of the authors of the study, Prof. Neal K. Devaraj from the University of California, San Diego and Alexander Harjung, a graduate student working in Prof. Devaraj's Lab.
Speaking of the team's motivation to develop this novel method, Prof. Devaraj said, "The reconstitution of membrane proteins into artificial systems has been a long-standing problem in artificial cell research.
"Membrane proteins are often insoluble in water, which makes them difficult to work with. Natural cells have complex systems ensuring these proteins can be efficiently inserted into cell membranes."
Harjung added, "For artificial cells, it would be very challenging to reconstitute these membrane insertion systems, which is why we saw a need for the development of a much simpler system for artificial cells to gain the ability to functionalize their own cell membrane."
For the study, the researchers aimed to functionalize the cell membrane to enable protein transport across the membrane and assemble them into tissue-like structures afterward.
Working with α-hemolysin
Biological channels typically use ion channels and transporters to exchange substances across the membrane. In artificial cells, this interaction has to be replicated manually.
Prof. Devaraj explained, "The researcher can change the membrane composition to achieve this, which is very different from how natural cells interact with their environment.
"To overcome this problem, we developed a method with which you can encode modification of the outer membrane, and thereby interact with the external environment, into the artificial cell genome."
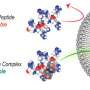
To do so, the researchers chose a pore-forming protein called α-hemolysin. This is a protein produced by Staphylococcus aureus, the bacteria responsible for causing staph infections. It is technically termed a toxin since it forms holes in cell membranes.
Explaining the reasoning behind choosing this protein, Harjung said, "Many researchers are already familiar with it due to its widespread use in artificial cells and nanopore sequencing.
"It has the unique ability to be expressed as a soluble monomer, which upon contact with a lipid bilayer (cell membrane) spontaneously assembles into a transmembrane protein."
The researchers not only used the α-Hemolysin as a pore-forming protein but also modified the artificial cells to produce the protein themselves. By having a self-sustaining system, the researchers do not need to add the protein each time.

Inserting peptides and producing α-hemolysin mutations
To enhance the functionality of α-hemolysin and achieve better control of the pore-forming process, the researchers decided to modify it.
In particular, their focus was to modify the membrane translocating loop of the protein, which is the part of the protein that plays a part in the translocation.
They tested peptides of different lengths and compositions. Peptides are short chains of amino acids, which are the building blocks of proteins. They used flexible linkers, short amino acid chains, that act like bridges to facilitate interaction or movement between different parts of the protein.
This step improves the peptide's accessibility once the protein gets embedded in the cell membrane.
"The flexible linker ensures the inserted peptide is accessible after translocation across the membrane. By varying the length of the linker we were able to understand more about the size of the peptide insert that could be translocated with our system," explained Prof. Devaraj.
The researchers tested various peptides. His-tag peptides—short sequences of histidine amino acids—were used to track the movement of the α-hemolysin as it travels and embeds into the cell membrane.
Next, the researchers used two biologically active peptides, Somatostatin-14 and GLP-1, as inserts in α-hemolysin to test the translocation.
To validate their findings, the researchers used several methods, including GUV binding and leakage assay for testing peptide-membrane interactions, cryo-electron microscopy to examine protein structure, lipid bilayer channel recordings to assess pore formation, and antibody recognition experiments to confirm the peptide translocation.
Successful protein translocation
The modified α-hemolysin successfully traveled to the cell membrane and embedded itself. Following this, the peptide inserts could successfully translocate across the membrane, demonstrating protein transport.
Peptides containing up to 50 amino acids could be inserted into α-hemolysin without disrupting pore formation, membrane insertion, and protein functionality.
The researchers further found that the translocated peptides remained accessible on the external side of the membrane. This suggests they could be used for assembling tissue-like structures, as their accessibility allows for further interactions and organization in the external environment.
Harjung explained this, saying, "The system enables the assembly of tissue-like structures based on electrostatic interactions.
"By generating one population of artificial cells that translocate negatively charged peptides across their membrane and another population of artificial cells that translocate positively charged peptides, we can create a tissue-like structure because artificial cells with a negatively charged outer membrane will bind to artificial cells with a positively charged membrane."
Drug delivery and artificial tissues
The researchers also added a system to detect if the cells can communicate with each other, where cells produce a visible (fluorescent) signal when they receive a signal from other cells. This could help with the creation of more complex and functional artificial tissues for future applications.
With the possibility of developing artificial tissues and potential drug delivery systems, the novel method demonstrates a pivotal step in cell research.
"With the development of biologics, methods for the efficient delivery of biological macromolecules into living cells have increasing importance in medicine," mentioned Prof. Devaraj.
Harjung added, "A better understanding of membrane translocation could lead to the development of tools for the delivery of macromolecular therapeutics across lipid membranes and into living cells."
Written for you by our author —this article is the result of careful human work. We rely on readers like you to keep independent science journalism alive. If this reporting matters to you, please consider a (especially monthly). You'll get an ad-free account as a thank-you.
More information: Alexander Harjung et al, Encoding extracellular modification of artificial cell membranes using engineered self-translocating proteins, Nature Communications (2024). ,
Journal information: Nature Communications
© 2024 Science X Network