Study discovers a nano-switch mechanism controlled by a single hydrogen atom in all living organisms

A group of researchers in Japan has revealed, for the first time, a mechanism for controlling the potential of an electron carrier protein in the redox reaction that all organisms need to obtain energy. was published in the online edition of eLife on November 15, 2024.
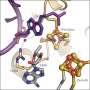
Based on experiments, the precise 3D structure of the protein, including hydrogen atoms, was determined, and theoretical calculations using this data visualized the electronic structure of the iron-sulfur cluster.
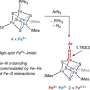
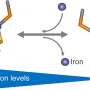
The researchers report that the electric potential of the iron-sulfur cluster changes dramatically depending on the presence or absence of a single hydrogen atom on an amino acid side chain, a so-called "nano-switch" mechanism.
The results not only deepen the scientific understanding of biological reactions but also provide a major clue to the future development of ultra-sensitive sensors for oxygen and nitric oxide and novel drugs.
Most reactions in living organisms involve electron transfer, which is called a redox reaction. For example, respiration and photosynthesis can be classified as redox reactions. Some proteins that assist in electron transfer contain iron and sulfur.
Ferredoxin is a small protein that contains iron-sulfur clusters and is known as the electron carrier in living organisms. Ferredoxin is a universal protein that is thought to be present in almost all living organisms; however, the mechanism by which ferredoxin stably carries electrons has remained a mystery to date.
-

Discovery of a nano-switch mechanism that controls the electric potential by a single hydrogen atom. Credit: eLife (2024). DOI: 10.7554/eLife.102506 -

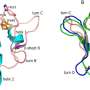
Structure around the iron-sulfur cluster. Credit: eLife (2024). DOI: 10.7554/eLife.102506
In the study, the researchers conducted experiments using the Ibaraki Biological Crystal Diffractometer (iBIX) at the Materials and Life Science Experimental Facility (MLF) in the Japan Proton Accelerator Research Complex (J-PARC) and determined the precise three-dimensional structure of a ferredoxin at the hydrogen atomic level in experiments using a neutron beam.
Visualizing hydrogen atoms in protein molecules using neutrons is extremely difficult, and less than 0.2% of the entire protein three-dimensional structure database has been reported.
Theoretical calculations using experimental geometry including hydrogen atoms were performed to elucidate the electronic structure of the iron-sulfur cluster in the ferredoxin.
The researchers found that an amino acid residue (aspartic acid 64) located far from the iron-sulfur cluster has a significant effect on the probability of electron transfer in the iron-sulfur cluster, and plays a role like a switch that controls the electron transfer in ferredoxin. Furthermore, the study found that the mechanism is universal in organisms.
More information: Kei Wada et al, Protonation/deprotonation-driven switch for the redox stability of low-potential [4Fe-4S] ferredoxin, eLife (2024).
Journal information: eLife
Provided by Osaka University