Ultrafast electron imaging captures never-before-seen nuclear motions in hydrocarbon molecules excited by light

The interactions between light and nitroaromatic hydrocarbon molecules have important implications for chemical processes in our atmosphere that can lead to smog and pollution. However, changes in molecular geometry due to interactions with light can be very difficult to measure because they occur at sub-Angstrom length scales and femtosecond time scales.
In a study, in the journal Â鶹ÒùÔºical Chemistry Chemical Â鶹ÒùÔºics, researchers used an ultrafast electron camera to image the motions of hydrocarbon molecules on scales 10,000 times smaller than the width of a human hair.
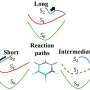
This ultra-precise and ultrafast imaging technique, supported by advanced computations, reveals a proton transfer step followed by an out-of-plane twisting motion as key components of energy relaxation. (Relaxation is the process by which the molecule moves from an excited, high-energy state to a lower energy ground state after absorbing light.)
Previous studies have proposed various ways that hydrocarbon molecules may relax after interacting with light. However, scientists lacked experimental data to verify which process occurs.
In this research, scientists used the relativistic ultrafast electron diffraction (UED) instrument to observe the relaxation of photoexcited o-nitrophenol. Then, they used a genetic structure fitting algorithm to extract new information about small changes in the molecular shape from the UED data that were imperceptible in previous studies.
Specifically, the experiment resolved the key processes in the relaxation of o-nitrophenol: proton transfer and deplanarization (i.e., a rotation of part of the molecule out of the molecular plane). Ab-initio multiple spawning simulations confirmed the experimental findings.
The researchers were able to identify a key relaxation pathway involving proton transfer and molecular "twisting." This result lays the groundwork for studies of more complex molecules that scientists believe undergo similar interactions. It will also help researchers better understand how pollution forms.
More information: J. P. F. Nunes et al, Photo-induced structural dynamics of o-nitrophenol by ultrafast electron diffraction, Â鶹ÒùÔºical Chemistry Chemical Â鶹ÒùÔºics (2024).
Journal information: Â鶹ÒùÔºical Chemistry Chemical Â鶹ÒùÔºics
Provided by US Department of Energy