Light-activated catalyst enables chiral synthesis with higher yields and less waste

Chemists and chemical engineers at California Institute of Technology, working with a pair of colleagues from the University of Pittsburgh, have developed a new light-activated catalyst that can be used for photoinduced deracemization of tertiary and secondary alkyl halides. In their paper in the journal Nature, the group describes how the new catalyst works and possible uses for it.
Wenzheng Fan and Guosheng Liu with the University of Chinese Academy of Sciences outline the difficulties chemists face when trying to produce single enantiomers from chiral molecules and then give an overview of the work done by the team on this new effort in a piece they have had published in the same issue.
One of the challenges in chemistry involves the synthesis of chiral molecules, which form mirror-image isomers called enantiomers. Typically, only one is desired because the other does not produce the same results when used in an application. One of the most common ways of dealing with the problem is to separate the mirror images into two groups, and then to toss out the group that is not useful.
Unfortunately, such an approach is very wasteful, which is why chemists have been working to find a way to get chiral molecules to form just the desired mirror enantiomer. In this new approach, the team working at CIT has found a way to do this using a light-activated catalyst.
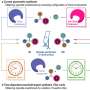
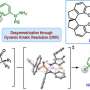
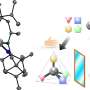
The new approach involves binding a copper chloride to a chiral phosphine ligand—one that can modulate its reactivity. Next, light is applied, activating the catalyst—it sets off a transfer reaction between single electrons and the halide substrate, breaking the bonds holding them together. The result is the generation of radical intermediates.
In the next part of the reaction, chloride is transferred from the copper complex to the radical produced earlier. That leaves a single chiral phosphine ligand, leading the rest of the reaction to produce the single-enantiomer product that was the goal of the entire reaction.
The team demonstrated their catalyst using a variety of alkyl halides, and in all cases, they found it produced much higher yields than in separation approaches.
More information: Feng Zhong et al, Photoinduced copper-catalysed deracemization of alkyl halides, Nature (2025).
Wenzheng Fan et al, Light wins uphill battle to solve enduring problem in organic synthesis, Nature (2025).
Journal information: Nature
© 2025 Science X Network