Scientists decode water's key role in platinum-catalyzed biomass conversion

Sadie Harley
scientific editor

Robert Egan
associate editor

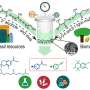
Scientists have delineated the critical atomic-scale mechanism that enables water to significantly enhance platinum (Pt)-catalyzed biomass conversion, according to a study published in the .
Biomass, one of the planet's most abundant renewable resources, can be catalytically transformed into fuels and chemicals to replace fossil-derived products—a key pathway for achieving global carbon neutrality goals.
A core step in this process involves processing furanic compounds: pivotal platform molecules whose conversion demands the selective cleavage of C-O bonds in their stable furan rings. This cleavage is essential to produce high-value chain alcohols, carboxylic acids, and amines.
Previous experiments have shown that Pt-catalyzed furan ring-opening hydrogenation proceeds far faster in water than in organic solvents, with notably different product selectivity. Yet the atomic-scale mechanism driving water's dramatic catalytic enhancement has long remained unclear—until now.
To resolve this mystery, a research team led by Prof. Zhang Jian from the Ningbo Institute of Materials Technology and Engineering (NIMTE) of the Chinese Academy of Sciences collaborated with Prof. William A. Goddard III from the California Institute of Technology (Caltech).
By combining isotope kinetic experiments with quantum mechanics calculations, the team identified water's dual role: acting as both a "proton shuttle" and a "nucleophile."
Their findings outline a detailed "water-mediated pathway." In this mechanism, water directly participates in the reductive scission of C−O bonds by assisting proton transfer—effectively activating a reaction pathway with a low energy barrier. Additionally, water molecules function as nucleophiles, attacking the C(2) carbon atom in the furanic compound. This attack triggers hydroxyl migration in the reaction intermediate, which is then sequentially hydrogenated to form chain alcohols.
Notably, hydronium ions (H3O+) spontaneously form at the interface between the platinum catalyst and water throughout the catalytic cycle. These ions, the team found, indirectly influence both the reaction mechanism and its kinetics.
"Understanding the catalytic role of solvents provides critical insights for advancing complex liquid-solid catalytic processes," said Prof. Zhang, a corresponding author of the study. "This knowledge will allow us to design more efficient biomass conversion processes, supporting the sustainable production of chemicals."
The research offers theoretical support for scaling up the green, industrial production of bio-based chemicals—materials seen as central to reducing reliance on fossil fuels and cutting carbon emissions.
More information: Mingxin Lv et al, The Water-Mediated Reaction Pathway for Catalytic Opening of the Furanic Ring on Platinum Catalysts, Journal of the American Chemical Society (2025).
Journal information: Journal of the American Chemical Society
Provided by Chinese Academy of Sciences