Exotic ring design breaks symmetry rules, unlocking new photocatalyst potential

Sadie Harley
scientific editor

Robert Egan
associate editor
![Three types of hetero[8]circulenes synthesized to date. Credit: The University of Osaka Novel unsymmetrical molecule produces perfect photocatalyst potential](https://scx1.b-cdn.net/csz/news/800a/2025/novel-unsymmetrical-mo.jpg)
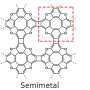
Life as we know it is based on organic molecules. In these molecules, carbon and hydrogen atoms are linked into a fascinating array of structures, such as chains or rings. One special class of organic molecules, hetero[8]circulenes, can behave in interesting ways because of their ring of eight atoms, and have many applications, including electronic devices responsible for controlling and detecting light.
However, creating these molecules through planned chemical reactions, or the synthetic route, is difficult. This is because of the resources needed, such as time and materials, to form this class of molecules.
As a result, only three types of hetero[8]circulenes have been made to date. These three types of molecules are symmetrical, i.e., if you draw a line through each molecule, both halves are mirror images of each other, or if you rotate each molecule, it looks the same.
However, the creation process may have become slightly easier because of the work of researchers at the University of Osaka.
The resulting , "Electrochemical cascade access to hetero[8]circulenes as potent organophotocatalysts for diverse C–X bond formations" in Nature Communications, highlights a new exotic ring design—dioxaza[8]circulene—as a gateway to a new class of structures. These findings push the boundaries of symmetry within hetero[8]circulenes.
The team used electric current to drive an electrochemical synthesis, or reaction, to form six links between atoms at the same time. This then created a structure of five hexagons and three pentagons, with this novel structure being an unsymmetrical hetero[8]circulene, dioxaza[8]circulene.
![Electrolytic domino synthesis of novel (type IV) hetero[8]circulene. Credit: The University of Osaka Novel unsymmetrical molecule produces perfect photocatalyst potential](https://scx1.b-cdn.net/csz/news/800a/2025/novel-unsymmetrical-mo-1.jpg)
"Removing the limitation of symmetry enabled us to unlock a new class of materials," says main author, Mohamed S. H. Salem. "The new method we developed to achieve this, from our unsymmetrical hetero[8]circulene, has only two steps and is simple and easy to use."
The benefits of the new, eco-friendly method are that it generates only water as a byproduct, and the materials needed for the reaction are not specialized and can be bought commercially. Overall, the method is efficient, by producing the dioxaza[8]circulene in yields up to 83%, i.e., 83% of the amount that can be produced in principle.
The new dioxaza[8]circulene molecule is not just an odd molecule without purpose, as it has unique properties that make it practically useful.
"Special tests showed us that in the dioxaza[8]circulene structure, the way electrons move and the way the molecule responds to light and electricity are all unusual," explains Shinobu Takizawa, senior author. "As a result, the dioxaza[8]circulene can act as an organic photocatalyst."
![Transition-metal-free cross-coupling reaction promoted by hetero[8]circulene photocatalyst. Credit: The University of Osaka Novel unsymmetrical molecule produces perfect photocatalyst potential](https://scx1.b-cdn.net/csz/news/800a/2025/novel-unsymmetrical-mo-2.jpg)
A photocatalyst can speed up any chemical reaction triggered by light, which is a sustainable and inexpensive process for material creation. The dioxaza[8]circulene was deemed to be a potent enough photocatalyst, meaning it has potential to help create compounds containing links between carbon and other atoms (carbon, boron, sulfur, and phosphorus) at a 97% yield, without requiring the use of transition metals.
Therefore, in creating the novel unsymmetrical hetero[8]circulene structure, the researchers have opened up a new avenue for making other materials in a safe and easy way, particularly given the unusual, but exciting, properties of dioxaza[8]circulene.
More information: Ahmed S. Gabr et al, Electrochemical cascade access to hetero[8]circulenes as potent organophotocatalysts for diverse C–X bond formations, Nature Communications (2025).
Journal information: Nature Communications
Provided by University of Osaka